Synthesis, Characterization, and Properties of [4]Cyclo-2,7-pyrenylene: Effects of Cyclic Structure on the Electronic Properties of Pyrene Oligomers†
Takahiro Iwamoto
Institute for Chemical Research, Kyoto University, Uji 611-0011 (Japan)
Search for more papers by this authorDr. Eiichi Kayahara
Institute for Chemical Research, Kyoto University, Uji 611-0011 (Japan)
CREST, Science and Technology Agency, Tokyo (Japan)
Search for more papers by this authorDr. Nobuhiro Yasuda
Japan Synchrotron Radiation Research Institute, Hyogo 679-5198 (Japan)
Search for more papers by this authorProf. Dr. Toshiyasu Suzuki
Institute for Molecular Science, Okazaki 444-8787 (Japan)
CREST, Science and Technology Agency, Tokyo (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Shigeru Yamago
Institute for Chemical Research, Kyoto University, Uji 611-0011 (Japan)
CREST, Science and Technology Agency, Tokyo (Japan)
Institute for Chemical Research, Kyoto University, Uji 611-0011 (Japan)Search for more papers by this authorTakahiro Iwamoto
Institute for Chemical Research, Kyoto University, Uji 611-0011 (Japan)
Search for more papers by this authorDr. Eiichi Kayahara
Institute for Chemical Research, Kyoto University, Uji 611-0011 (Japan)
CREST, Science and Technology Agency, Tokyo (Japan)
Search for more papers by this authorDr. Nobuhiro Yasuda
Japan Synchrotron Radiation Research Institute, Hyogo 679-5198 (Japan)
Search for more papers by this authorProf. Dr. Toshiyasu Suzuki
Institute for Molecular Science, Okazaki 444-8787 (Japan)
CREST, Science and Technology Agency, Tokyo (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Shigeru Yamago
Institute for Chemical Research, Kyoto University, Uji 611-0011 (Japan)
CREST, Science and Technology Agency, Tokyo (Japan)
Institute for Chemical Research, Kyoto University, Uji 611-0011 (Japan)Search for more papers by this authorThis work was supported by the Core Research for Evolutional Science and Technology (CREST) from the Japan Science and Technology Agency of Japan. T.I. acknowledges the receipt of a JSPS Fellowship for Young Scientists. Computation time was provided by the Super Computer Laboratory, Institute for Chemical Research, Kyoto University. We thank Dr. Hikaru Takaya (Institute for Chemical Research, Kyoto University) and Dr. Kunihisa Sugimoto (JASRI) for their guidance and valuable discussions on the X-ray crystallographic analysis.
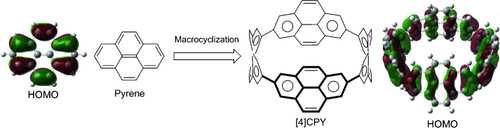
Graphical Abstract
Changing the landscape: A cyclic tetramer of pyrene, [4]cyclo-2,7-pyrenylene ([4]CPY), was synthesized by the platinum-mediated cyclotetramerization and subsequent dehydrogenation. DFT calculations and electrochemical analyses showed that the electronic structure of [4]CPY was completely altered from that of pyrene and linear oligopyrenes. The results clearly show there is modulation of the topology of molecular orbitals upon formation of a cyclic structure.
Abstract
A cyclic tetramer of pyrene, [4]cyclo-2,7-pyrenylene ([4]CPY), was synthesized from pyrene in six steps and 18 % overall yield by the platinum-mediated assembly of pyrene units and subsequent reductive elimination of platinum. The structures of the two key intermediates were unambiguously determined by X-ray crystallographic analysis. DFT calculations showed that the topology of the frontier orbitals in [4]CPY was essentially the same as those in [8]cycloparaphenylene ([8]CPP), and that all the pyrene units were fully conjugated. The electrochemical analyses proved the electronic properties of [4]CPY to be similar to those of [8]CPP. The results are in sharp contrast to those obtained for the corresponding linear oligomers of pyrene in which each pyrene unit was electronically isolated. The results clearly show a novel effect of the cyclic structure on cyclic π-conjugated molecules.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201403624_sm_miscellaneous_information.pdf1.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aL. T. Scott, Angew. Chem. 2003, 115, 4265–4267; Angew. Chem. Int. Ed. 2003, 42, 4133–4135;
- 1bT. Kawase, H. Kurata, Chem. Rev. 2006, 106, 5250–5273;
- 1cK. Tahara, Y. Tobe, Chem. Rev. 2006, 106, 5274–5290;
- 1dB. D. Steinberg, L. T. Scott, Angew. Chem. 2009, 121, 5504–5507;
10.1002/ange.200901025 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 5400–5402;
- 1eD. Eisenberg, R. Shenhar, M. Rabinovitz, Chem. Soc. Rev. 2010, 39, 2879–2890;
- 1fM. Iyoda, J. Yamakawa, M. J. Rahman, Angew. Chem. 2011, 123, 10708–10740;
10.1002/ange.201006198 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 10522–10553.
- 2
- 2aT. J. Sisto, R. Jasti, Synlett 2012, 483–489;
- 2bH. Omachi, Y. Segawa, K. Itami, Acc. Chem. Res. 2012, 45, 1378–1389;
- 2cS. Yamago, E. Kayahara, T. Iwamoto, Chem. Rec. 2014, 14, 84–100.
- 3
- 3aR. Jasti, J. Bhattacharjee, J. B. Neaton, C. R. Bertozzi, J. Am. Chem. Soc. 2008, 130, 17646–17647;
- 3bT. J. Sisto, M. R. Golder, E. S. Hirst, R. Jasti, J. Am. Chem. Soc. 2011, 133, 15800–15802;
- 3cE. R. Darzi, T. J. Sisto, R. Jasti, J. Org. Chem. 2012, 77, 6624–6628;
- 3dJ. Xia, J. W. Bacon, R. Jasti, Chem. Sci. 2012, 3, 3018–3021;
- 3eJ. Xia, R. Jasti, Angew. Chem. 2012, 124, 2524–2526; Angew. Chem. Int. Ed. 2012, 51, 2474–2476;
- 3fP. J. Evans, E. R. Darzi, R. Jasti, Nat. Chem. 2014, 6, 404–408.
- 4
- 4aH. Takaba, H. Omachi, Y. Yamamoto, J. Bouffard, K. Itami, Angew. Chem. 2009, 121, 6228–6232;
10.1002/ange.200902617 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 6112–6116;
- 4bH. Omachi, S. Matsuura, Y. Segawa, K. Itami, Angew. Chem. 2010, 122, 10400–10403; Angew. Chem. Int. Ed. 2010, 49, 10202–10205;
- 4cY. Segawa, P. Šenel, S. Matsuura, H. Omachi, K. Itami, Chem. Lett. 2011, 40, 423–425;
- 4dY. Segawa, S. Miyamoto, H. Omachi, S. Matsuura, P. Šenel, T. Sasamori, N. Tokitoh, K. Itami, Angew. Chem. 2011, 123, 3302–3306; Angew. Chem. Int. Ed. 2011, 50, 3244–3248;
- 4eY. Ishii, Y. Nakanishi, H. Omachi, S. Matsuura, K. Matsui, H. Shinohara, Y. Segawa, K. Itami, Chem. Sci. 2012, 3, 2340–2345;
- 4fF. Sibbel, K. Matsui, Y. Segawa, A. Studer, K. Itami, Chem. Commun. 2014, 50, 954–956.
- 5
- 5aS. Yamago, Y. Watanabe, T. Iwamoto, Angew. Chem. 2010, 122, 769–771;
10.1002/ange.200905659 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 757–759;
- 5bT. Iwamoto, Y. Watanabe, Y. Sakamoto, T. Suzuki, S. Yamago, J. Am. Chem. Soc. 2011, 133, 8354–8361;
- 5cE. Kayahara, Y. Sakamoto, T. Suzuki, S. Yamago, Org. Lett. 2012, 14, 3284–3287;
- 5dE. Kayahara, T. Iwamoto, T. Suzuki, S. Yamago, Chem. Lett. 2013, 42, 621–623;
- 5eE. Kayahara, V. K. Patel, S. Yamago, J. Am. Chem. Soc. 2014, 136, 2284–2287.
- 6
- 6aM. Fujitsuka, D. W. Cho, T. Iwamoto, S. Yamago, T. Majima, Phys. Chem. Chem. Phys. 2012, 14, 14585–14588;
- 6bM. Fujitsuka, T. Iwamoto, E. Kayahara, S. Yamago, T. Majima, ChemPhysChem 2013, 14, 1570–1572;
- 6cY. Segawa, A. Fukazawa, S. Matsuura, H. Omachi, S. Yamaguchi, S. Irle, K. Itami, Org. Biomol. Chem. 2012, 10, 5979–5984;
- 6dC. Camacho, T. A. Niehaus, K. Itami, S. Irle, Chem. Sci. 2013, 4, 187–195.
- 7
- 7aK. Masui, Y. Segawa, T. Namikawa, K. Kamada, K. Itami, Chem. Sci. 2013, 4, 84–88;
- 7bH. Omachi, T. Nakayama, E. Takahashi, Y. Segawa, K. Itami, Nat. Chem. 2013, 5, 572–576;
- 7cE. Kayahara, T. Iwamoto, H. Takaya, T. Suzuki, M. Fujitsuka, T. Majima, N. Yasuda, N. Matsuyama, S. Seki, S. Yamago, Nat. Commun. 2013, 4, 2694.
- 8
- 8aT. Iwamoto, Y. Watanabe, T. Sadahiro, T. Haino, S. Yamago, Angew. Chem. 2011, 123, 8492–8494; Angew. Chem. Int. Ed. 2011, 50, 8342–8344;
- 8bT. Iwamoto, Y. Watanabe, H. Takaya, T. Haino, N. Yasuda, S. Yamago, Chem. Eur. J. 2013, 19, 14061–14068.
- 9
- 9aH. Omachi, Y. Segawa, K. Itami, Org. Lett. 2011, 13, 2480–2483;
- 9bJ. Xia, M. R. Golder, M. E. Foster, B. M. Wong, R. Jasti, J. Am. Chem. Soc. 2012, 134, 19709–19715;
- 9cK. Matsui, Y. Segawa, K. Itami, Org. Lett. 2012, 14, 1888–1891;
- 9dA. Yagi, Y. Segawa, K. Itami, J. Am. Chem. Soc. 2012, 134, 2962–2965;
- 9eT. Nishiuchi, X. Feng, V. Enkelmann, M. Wanger, K. Müllen, Chem. Eur. J. 2012, 18, 16621–16625;
- 9fF. E. Golling, M. Quernheim, M. Wagner, T. Nishiuchi, K. Müllen, Angew. Chem. 2014, 126, 1551–1554;
10.1002/ange.201309104 Google ScholarAngew. Chem. Int. Ed. 2014, 53, 1525–1528.
- 10
- 10aS. Hitosugi, W. Nakanishi, T. Yamasaki, H. Isobe, Nat. Commun. 2011, 2, 492;
- 10bS. Hitosugi, T. Yamasaki, H. Isobe, J. Am. Chem. Soc. 2012, 134, 12442–12445;
- 10cT. Matsuno, S. Kamata, S. Hitosugi, H. Isobe, Chem. Sci. 2013, 4, 3179–3183.
- 11E. Kayahara, T. Kouyama, T. Kato, H. Takaya, N. Yasuda, S. Yamago, Angew. Chem. 2013, 125, 13967–13971;
10.1002/ange.201306881 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 13722–13726.
- 12T. M. Figueira-Duarte, K. Müllen, Chem. Rev. 2011, 111, 7260–7314.
- 13
- 13aG. J. Bodwell, D. O. Miller, R. J. Vermeij, Org. Lett. 2001, 3, 2093–2096;
- 13bB. L. Merner, L. N. Dawe, G. J. Bodwell, Angew. Chem. 2009, 121, 5595–5599;
10.1002/ange.200806363 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 5487–5491.
- 14A. Yagi, V. Venkataramana, Y. Segawa, K. Itami, Chem. Commun. 2014, 50, 957–959.
- 15M. Kreyenschmidt, M. Baumgarten, N. Tyutyulkov, K. Müllen, Angew. Chem. 1994, 106, 2062–2064; Angew. Chem. Int. Ed. Engl. 1994, 33, 1957–1959.
- 16R. C. Haddon, Science 1993, 261, 1545–1550.
- 17
- 17aD. Rausch, C. Lambert, Org. Lett. 2006, 8, 5037–5040;
- 17bD. M. Connor, S. D. Allen, D. M. Collard, C. L. Liotta, D. A. Schiraldi, J. Org. Chem. 1999, 64, 6888–6890.
- 18CCDC 972620 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 19S. Shekhar, J. F. Hartwig, J. Am. Chem. Soc. 2004, 126, 13016–13027.
- 20CCDC 972621 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 21Use of 2,7-bistrimethylstannylpyrene as a precursor of [4]CPY was unsuccessful because of its low solubility to common solvents.
- 22See the Supporting Information for the physical properties of [4]CPHY. The electronic structure of [4]CPHY was virtually identical to that of [8]CPP.