A Metal-Free Strategy to Release Chemisorbed H2 from Hydrogenated Boron Nitride Nanotubes†
Lisa Roy
Raman Centre for Atomic Molecular and Optical Sciences, Indian Association for the Cultivation of Science, 2A & 2B, Raja S.C. Mullick Road, Kolkata, 700032 (India)
Search for more papers by this authorSourav Bhunya
Raman Centre for Atomic Molecular and Optical Sciences, Indian Association for the Cultivation of Science, 2A & 2B, Raja S.C. Mullick Road, Kolkata, 700032 (India)
Search for more papers by this authorCorresponding Author
Dr. Ankan Paul
Raman Centre for Atomic Molecular and Optical Sciences, Indian Association for the Cultivation of Science, 2A & 2B, Raja S.C. Mullick Road, Kolkata, 700032 (India)
Raman Centre for Atomic Molecular and Optical Sciences, Indian Association for the Cultivation of Science, 2A & 2B, Raja S.C. Mullick Road, Kolkata, 700032 (India)Search for more papers by this authorLisa Roy
Raman Centre for Atomic Molecular and Optical Sciences, Indian Association for the Cultivation of Science, 2A & 2B, Raja S.C. Mullick Road, Kolkata, 700032 (India)
Search for more papers by this authorSourav Bhunya
Raman Centre for Atomic Molecular and Optical Sciences, Indian Association for the Cultivation of Science, 2A & 2B, Raja S.C. Mullick Road, Kolkata, 700032 (India)
Search for more papers by this authorCorresponding Author
Dr. Ankan Paul
Raman Centre for Atomic Molecular and Optical Sciences, Indian Association for the Cultivation of Science, 2A & 2B, Raja S.C. Mullick Road, Kolkata, 700032 (India)
Raman Centre for Atomic Molecular and Optical Sciences, Indian Association for the Cultivation of Science, 2A & 2B, Raja S.C. Mullick Road, Kolkata, 700032 (India)Search for more papers by this authorA.P. thanks the DST FASTRACK (NO-SR/FT/CS-118/2011) for providing research funds. L.R. and S.B. acknowledge research fellowships of the CSIR (India).
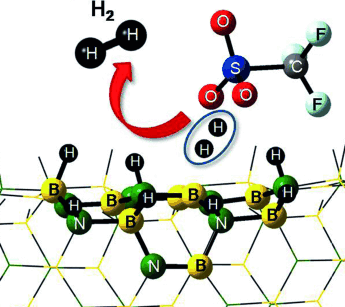
Graphical Abstract
Abstract
Chemisorbed hydrogen on boron nitride nanotubes (BNNT) can only be released thermally at very high temperatures above 350 °C. However, no catalyst has been identified that could liberate H2 from hydrogenated BN nanotubes under moderate conditions. Using different density functional methods we predict that the desorption of chemisorbed hydrogen from hydrogenated BN nanotubes can be facilitated catalytically by triflic acid at low free-energy activation barriers and appreciable rates under metal free conditions and mildly elevated temperatures (40–50 °C). Our proposed mechanism shows that the acid is regenerated in the process and can further facilitate similar catalytic release of H2, thus suggesting all the chemisorbed hydrogen on the surface of the hydrogenated nanotube can be released in the form of H2. These findings essentially raise hope for the development of a sustainable chemical hydrogen storage strategy in BN nanomaterials.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201403610_sm_miscellaneous_information.pdf5.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aThe Hydrogen Economy: NRC and NAE, The National Academic press, Washington, DC, 2004;
- 1bTargets for Onboard Hydrogen Storage Systems for Light-Duty Vehicles, U.S. DOE, September 2009. http://energy.gov/sites/prod/files/2014/03/f11/targets_onboard_hydro_storage_explanation.pdf.
- 2
- 2aT. Yildirim, M. R. Hartman, Phys. Rev. Lett. 2005, 95, 215504;
- 2bH. Lee, J.-w. Lee, D. Y. Kim, J. Park, Y.-T. Seo, H. Zeng, I. L. Moudrakovski, C. I. Ratcliffe, J. A. Ripmeester, Nature 2005, 434, 743–746;
- 2cA. C. Dillon, K. M. Jones, T. A. Bekkedahl, C. H. Kiang, D. S. Bethune, M. J. Heben, Nature 1997, 386, 377–379;
- 2dY. Zhao, Y.-H. Kim, A. C. Dillon, M. J. Heben, S. B. Zhang, Phys. Rev. Lett. 2005, 94, 155504;
- 2eB. Bogdanović, M. Felderhoff, S. Kaskel, A. Pommerin, K. Schlichte, F. Schüth, Adv. Mater. 2003, 15, 1012–1015;
- 2fZ. Xiong, C. K. Yong, G. Wu, P. Chen, W. Shaw, A. Karkamkar, T. Autrey, M. O. Jones, S. R. Johnson, P. P. Edwards, W. I. F. David, Nat. Mater. 2008, 7, 138–141.
- 3
- 3aT. B. Marder, Angew. Chem. Int. Ed. 2007, 46, 8116–8118; Angew. Chem. 2007, 119, 8262–8264;
- 3bC. W. Hamilton, R. T. Baker, A. Staubitz, I. Manners, Chem. Soc. Rev. 2009, 38, 279–293;
- 3cA. Staubitz, A. P. M. Robertson, I. Manners, Chem. Rev. 2010, 110, 4079–4124;
- 3dG. Alcaraz, S. Sabo-Etienne, Angew. Chem. Int. Ed. 2010, 49, 7170–7179; Angew. Chem. 2010, 122, 7326–7335.
- 4
- 4aA. D. Sutton, A. K. Burrell, D. A. Dixon, E. B. Garner III, J. C. Gordon, T. Nakagawa, K. C. Ott, J. P. Robinson, M. Vasiliu, Science 2011, 331, 1426–1429;
- 4bG. R. Whittell, I. Manners, Angew. Chem. Int. Ed. 2011, 50, 10288–10289; Angew. Chem. 2011, 123, 10470–10472.
- 5B. E. Smith, R. L. Richards, W. E. Newton, Catalysts for Nitrogen fixation, Springer, Dordrecht, 2004.
10.1007/978-1-4020-3611-8 Google Scholar
- 6R. Ma, Y. Bando, H. Zhu, T. Sato, C. Xu, D. Wu, J. Am. Chem. Soc. 2002, 124, 7672–7673.
- 7C. Tang, Y. Bando, X. Ding, S. Qi, D. Golberg, J. Am. Chem. Soc. 2002, 124, 14550–14551.
- 8S. H. Lim, J. Luo, W. Ji, J. Lin, Catal. Today 2007, 120, 346–350.
- 9aD. Golberg, Y. Bando, Y. Huang, T. Terao, M. Mitome, C. Tang, C. Zhi, ACS Nano 2010, 4, 2979–2993;
- 9bD. Golberg, Y. Bando, C. Tang, C. Zhi, Adv. Mater. 2007, 19, 2413–2432.
- 10
- 10aN. G. Chopra, R. J. Luyken, K. Cherrey, V. H. Crespi, M. L. Cohen, S. G. Louie, A. Zettl, Science 1995, 269, 966–967;
- 10bE. Bengu, L. D. Marks, Phys. Rev. Lett. 2001, 86, 2385–2387.
- 11
- 11aX. Wu, J. Yang, J. G. Hou, Q. Zhu, J. Chem. Phys. 2004, 121, 8481–8485;
- 11bS. S. Han, S. H. Lee, J. K. Kang, H. M. Lee, Phys. Rev. B 2005, 72, 113402.
- 12X. Chen, X. P. Gao, H. Zhang, Z. Zhou, W. K. Hu, G. L. Pan, H. Y. Zhu, T. Y. Yan, D. Y. Song, J. Phys. Chem. B 2005, 109, 11525–11529.
- 13
- 13aA. Nikitin, H. Ogasawara, D. Mann, R. Denecke, Z. Zhang, H. Dai, K. Cho, A. Nilsson, Phys. Rev. Lett. 2005, 95, 225507;
- 13bD. C. Elias, R. R. Nair, T. M. G. Mohiuddin, S. V. Morozov, P. Blake, M. P. Halsall, A. C. Ferrari, D. W. Boukhvalov, M. I. Katsnelson, A. K. Geim, K. S. Novoselov, Science 2009, 323, 610–613.
- 14K. S. Subrahmanyam, P. Kumar, U. Maitra, A. Govindaraj, K. P. S. S. Hembram, U. V. Waghmare, C. N. R. Rao, Proc. Natl. Acad. Sci. USA 2011, 108, 2674–2677.
- 15L. Roy, S. Mittal, A. Paul, Angew. Chem. Int. Ed. 2012, 51, 4152–4156; Angew. Chem. 2012, 124, 4228–4232.
- 16P. M. Zimmerman, Z. Zhang, C. B. Musgrave, Inorg. Chem. 2010, 49, 8724–8728.
- 17For recent reports on ammonia borane dehydrocoupling by transition-metal-based catalysts, see:
- 17aA. Paul, C. B. Musgrave, Angew. Chem. Int. Ed. 2007, 46, 8153–8156; Angew. Chem. 2007, 119, 8301–8304;
- 17bP. M. Zimmerman, A. Paul, Z. Zhang, C. B. Musgrave, Angew. Chem. Int. Ed. 2009, 48, 2201–2205; Angew. Chem. 2009, 121, 2235–2239;
- 17cH. Helten, B. Dutta, J. R. Vance, M. E. Sloan, M. F. Haddow, S. Sproules, D. Collison, G. R. Whittell, G. C. Lloyd-Jones, I. Manners, Angew. Chem. Int. Ed. 2013, 52, 437–440; Angew. Chem. 2013, 125, 455–458;
- 17dR. T. Baker, J. C. Gordon, C. W. Hamilton, N. J. Henson, P.-H. Lin, S. Maguire, M. Murugesu, B. L. Scott, N. C. Smythe, J. Am. Chem. Soc. 2012, 134, 5598–5609;
- 17eB. L. Conley, D. Guess, T. J. Williams, J. Am. Chem. Soc. 2011, 133, 14212–14215;
- 17fB. L. Conley, T. J. Williams, Chem. Commun. 2010, 46, 4815–4817.
- 18See the Supporting Information for details.
- 19
- 19aJ.-M. Denis, H. Forintos, H. Szelke, L. Toupet, T.-N. Pham, P.-J. Madec, A.-C. Gaumont, Chem. Commun. 2003, 54–55;
- 19bF. H. Stephens, R. T. Baker, M. H. Matus, D. J. Grant, D. A. Dixon, Angew. Chem. Int. Ed. 2007, 46, 746–749; Angew. Chem. 2007, 119, 760–763.
- 20S. Bhunya, A. Banerjee, R. Tripathi, N. N. Nair, A. Paul, Chem. Eur. J. 2013, 19, 17673–17678.
- 21aA. D. Becke, J. Chem. Phys. 1993, 98, 5648–5652;
- 21bJ.-D. Chai, M. Head-Gordon, Phys. Chem. Chem. Phys. 2008, 10, 6615–6620;
- 21cY. Zhao, N. E. Schultz, D. G. Truhlar, J. Chem. Theory Comput. 2006, 2, 364–382;
- 21dY. Zhao, D. G. Truhlar, Theor. Chem. Acc. 2008, 120, 215–241.
- 22
- 22a“Entropies of Condensed Phases and Complex Systems”: C. Spickermann, Springer Theses, 2010, Chap. 3, pp. 76–80, and references therein;
- 22bD. H. Wertz, J. Am. Chem. Soc. 1980, 102, 5316–5322;
- 22cH. Li, X. Wang, F. Huang, G. Lu, J. Jiang, Z.-X. Wang, Organometallics 2011, 30, 5233–5247.
- 23
- 23aV. Barone, M. Cossi, J. Phys. Chem. A 1998, 102, 1995–2001;
- 23bM. Cossi, N. Rega, G. Scalmani, V. Barone, J. Comput. Chem. 2003, 24, 669–681.
- 24V. M. Sánchez, M. Sued, D. A. Scherlis, J. Chem. Phys. 2009, 131, 174108.
- 25R. Buzzoni, S. Bordiga, G. Ricchiardi, G. Spoto, A. Zecchina, J. Phys. Chem. 1995, 99, 11937–11951.
- 26
- 26aD. C. Rosenfeld, S. Shekhar, A. Takemiya, M. Utsunomiya, J. F. Hartwig, Org. Lett. 2006, 8, 4179–4182;
- 26bZ. Li, J. Zhang, C. Brouwer, C.-G. Yang, N. W. Reich, C. He, Org. Lett. 2006, 8, 4175–4178, and references therein.
- 27J. S. Wang, R. A. Geanangel, Inorg. Chim. Acta 1988, 148, 185–190.
Citing Literature
Special Issue:Nanotechnology & Nanomaterials, Nanotoxicology & Nanomedicine
November 10, 2014
Pages 12430-12435