Multiple Phases of Molybdenum Carbide as Electrocatalysts for the Hydrogen Evolution Reaction†
This work was supported by University of Wyoming Start-up, and the School of Energy Resources. We are grateful for Dr. Erwin Sabio who assisted with the XPS studies.
Graphical Abstract
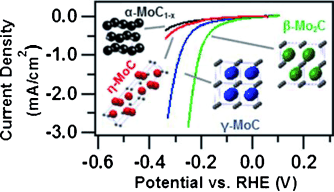
Four phases of Mo-C, including γ-MoC which was stabilized for the first time as a nanomaterial, were synthesized and investigated for their catalytic activity and stability in the hydrogen evolution reaction (HER). X-ray photoelectron spectroscopy and valence band studies were also conducted for the first time on γ-MoC.
Abstract
Molybdenum carbide has been proposed as a possible alternative to platinum for catalyzing the hydrogen evolution reaction (HER). Previous studies were limited to only one phase, β-Mo2C with an Fe2N structure. Here, four phases of Mo-C were synthesized and investigated for their electrocatalytic activity and stability for HER in acidic solution. All four phases were synthesized from a unique amine–metal oxide composite material including γ-MoC with a WC type structure which was stabilized for the first time as a phase pure nanomaterial. X-ray photoelectron spectroscopy (XPS) and valence band studies were also used for the first time on γ-MoC. γ-MoC exhibits the second highest HER activity among all four phases of molybdenum carbide, and is exceedingly stable in acidic solution.