Tandem Organocatalysis and Photocatalysis: An Anthraquinone-Catalyzed Indole-C3-Alkylation/Photooxidation/1,2-Shift Sequence†
We thank the University of Hamburg and the Fonds der Chemischen Industrie (FCI) for financial support.
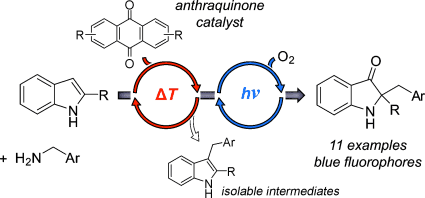
Graphical Abstract
Orthogonal reactivities: Anthraquinone derivatives catalyze the thermal C3- alkylation of indoles with benzylamines in sequence with a visible-light-driven photooxidation/1,2-shift reaction to provide new fluorescent 2,2-disubstituted indoline-3-one derivatives. Quinones function as H2 shuttles in the indole C3-alkylation with amines and the subsequent photooxidation of the intermediate 3-arylmethyl-1H-indoles is remarkably selective.
Abstract
Quinones exhibit orthogonal ground- and excited-state reactivities and are therefore highly suitable organocatalysts for the development of sequential catalytic processes. Herein, the discovery of an anthraquinone-catalyzed thermal indole-C3-alkylation with benzylamines is described, which can be combined sequentially with a new visible-light-driven catalytic photooxidation/1,2-shift reaction. The one-flask tandem process converts indoles into 3-benzylindole intermediates, which are further transformed into new fluorescent 2,2-disubstituted indoline-3-one derivatives.