Palladium-Catalyzed Asymmetric Amination of Allenyl Phosphates: Enantioselective Synthesis of Allenes with an Additional Unsaturated Unit†
Financial support from the National Basic Research Program of China (2011CB808700) and the National Natural Science Foundation of China (21232006) are greatly appreciated. We thank Hongwen Luo for reproducing the preparation of (S)-3 g (Table 5), and (S)-3 j and (S)-3 k (Table 6). Shengming Ma is a Qiu Shi Adjunct Professor at Zhejiang University.
Graphical Abstract
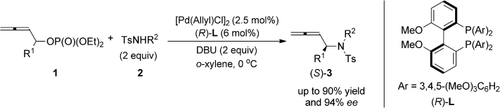
Chiral 2,3-allenyl amines with or without (an) additional CC double or triple bond(s) can be prepared through asymmetric Pd-catalyzed amination of allenyl phosphates. Under the optimized conditions, which involve the use of (R)-3,4,5-(MeO)3-MeOBIPHEP as the ligand and the reaction being performed at 0 °C, the products were obtained with up to 90 % yield and 88–94 % ee.
Abstract
A highly enantioselective Pd-catalyzed amination of allenyl phosphates generating 2,3-allenyl amines with central chirality has been developed. Under the optimized conditions, chiral 2,3-allenyl amines with or without (an) additional CC double or triple bond(s) have been prepared at 0 °C with up to 90 % yield and 94 % ee by identifying (R)-3,4,5-(MeO)3-MeOBIPHEP as the ligand.