Access to Oxoquinoline Heterocycles by N-Heterocyclic Carbene Catalyzed Ester Activation for Selective Reaction with an Enone†
Dr. Zhenqian Fu
Division of Chemistry & Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
Search for more papers by this authorDr. Ke Jiang
School of Biological Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
Search for more papers by this authorDr. Tingshun Zhu
Division of Chemistry & Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
Search for more papers by this authorProf. Dr. Jaume Torres
School of Biological Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Yonggui Robin Chi
Division of Chemistry & Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
Division of Chemistry & Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore)Search for more papers by this authorDr. Zhenqian Fu
Division of Chemistry & Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
Search for more papers by this authorDr. Ke Jiang
School of Biological Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
Search for more papers by this authorDr. Tingshun Zhu
Division of Chemistry & Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
Search for more papers by this authorProf. Dr. Jaume Torres
School of Biological Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Yonggui Robin Chi
Division of Chemistry & Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
Division of Chemistry & Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore)Search for more papers by this authorWe thank the Singapore National Research Foundation (NRF), Singapore Economic Development Board (EDB), GlaxoSmithKline (GSK), and Nanyang Technological University (NTU) for generous financial support. We thank Dr. Y. Li and Dr. R. Ganguly (X-ray structure, NTU) for their contribution. K.J. and J.T. acknowledge the financial support from the National Research Foundation grant NRF-CRP4-2008-02.
Graphical Abstract
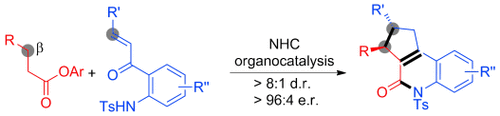
Under construction: A single-step enantioselective access to multicyclic oxoquinoline-type heterocycles is possible. The process takes advantage of the unique reaction patterns of esters under N-heterocyclic carbene (NHC) catalysis. It involves activation of the β-carbon atom of an ester as the key step with a subsequent chemoselective cascade reaction with amino enone substrates. Ts=4-toluenesulfonyl.
Abstract
Organocatalytic ester activation is developed for a highly selective cascade reaction between saturated esters and amino enones. The reaction involves activation of the β-carbon atom of the ester as a key step. This method allows a single-step access to multicyclic oxoquinoline-type heterocycles with high enantiomeric ratios.
References
- 1
- 1aK. Bernauer, G. Englert, W. Vetter, E. Weiss, Helv. Chim. Acta 1969, 52, 1886–1905;
- 1bM. Daudon, M. H. Mehri, M. M. Plat, E. W. Hagaman, F. M. Schell, E. Wenkert, J. Org. Chem. 1975, 40, 2838–2839;
- 1cL. E. Overman, G. M. Robertson, A. J. Robichaud, J. Org. Chem. 1989, 54, 1236–1238;
- 1dP. Selig, T. Bach, Angew. Chem. 2008, 120, 5160–5162; Angew. Chem. Int. Ed. 2008, 47, 5082–5084;
- 1eP. Selig, E. Herdtweck, T. Bach, Chem. Eur. J. 2009, 15, 3509–3525;
- 1fY. Hayashi, F. Inagaki, C. Mukai, Org. Lett. 2011, 13, 1778–1780;
- 1gH. M. Zhang, D. P. Curran, J. Am. Chem. Soc. 2011, 133, 10376–10378;
- 1hK. S. Feldman, J. F. Antoline, Org. Lett. 2012, 14, 934–937.
- 2H. Lee, J. Lee, S. I. Yang, Arch. Pharmacal Res. 2001, 24, 385–389.
- 3
- 3aE. E. Bosco, S. Kumar, F. Marchioni, J. Biesiada, M. Kordos, K. Szczur, J. Meller, W. Seibel, A. Mizrahi, E. Pick, M. D. Filippi, Y. Zheng, Chem. Biol. 2012, 19, 228–242;
- 3bY. Zheng, P. Jagtap, E. E. Bosco, J. Meller, M. Filippi, PCT Int. Appl. WO 2013123081, 2013.
- 4K. F. McDaniel, D. J. Kempf, U. S. Pat. Appl. Publ. US 20100168384, 2010.
- 5R. S. Upadhayaya, S. V. Lahore, A. Y. Sayyed, S. S. Dixit, P. D. Shinde, J. Chattopadhyaya, Org. Biomol. Chem. 2010, 8, 2180–2197.
- 6T. L. Andreana, S. S. Y. Cho, J. M. Graham, T. F. Gregory, H. R. J. Howard, B. E. Kornberg, S. S. Nikam, D. A. Pflum, PCT Int. Appl. WO 2004026864, 2004.
- 7
- 7aR. J. Brown, F. W. S. Carver, B. L. Hollingsworth, J. Chem. Soc. 1961, 4295–4298;
- 7bD. Desmaële, Tetrahedron Lett. 1996, 37, 1233–1236;
- 7cS. Jaroch, P. Holscher, H. Rehwinkel, D. Sulzle, G. Gurton, M. Hillmann, F. M. McDonald, Bioorg. Med. Chem. Lett. 2003, 13, 1981–1984;
- 7dL. Bharat, B. Pio, PCT Int. Appl. WO 2007041076, 2007;
- 7eB. Lagu, B. Pio, R. Lebedev, M. Yang, P. D. Pelton, Bioorg. Med. Chem. Lett. 2007, 17, 3497–3503;
- 7fM. Haddach, F. Pierre, PCT Int. Appl. WO 2011035019, 2011.
- 8For selected examples of enamine/iminium catalysis cascade reactions, see:
- 8aN. Halland, P. S. Aburel, K. A. Jørgensen, Angew. Chem. 2004, 116, 1292–1297;
10.1002/ange.200353364 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 1272–1277;
- 8bY. Huang, A. M. Walji, C. H. Larsen, D. W. C. MacMillan, J. Am. Chem. Soc. 2005, 127, 15051–15053;
- 8cD. Enders, M. R. M. Huttl, C. Grondal, G. Raabe, Nature 2006, 441, 861–863;
- 8dJ. Wang, H. Li, H. Xie, L. Zu, X. Shen, W. Wang, Angew. Chem. 2007, 119, 9208–9211; Angew. Chem. Int. Ed. 2007, 46, 9050–9053;
- 8eH. Xie, L. Zu, H. Li, J. Wang, W. Wang, J. Am. Chem. Soc. 2007, 129, 10886–10894;
- 8fP. Galzerano, F. Pesciaioli, A. Mazzanti, G. Bartoli, P. Melchiorre, Angew. Chem. 2009, 121, 8032–8034;
10.1002/ange.200903803 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 7892–7894;
- 8gA. Michrowska, B. List, Nat. Chem. 2009, 1, 225–228;
- 8hL. Albrecht, H. Jiang, G. Dickmeiss, B. Gschwend, S. G. Hansen, K. A. Jørgensen, J. Am. Chem. Soc. 2010, 132, 9188–9196;
- 8iC. Grondal, M. Jeanty, D. Enders, Nat. Chem. 2010, 2, 167–178;
- 8jS. B. Jones, B. Simmons, A. Mastracchio, D. W. C. MacMillan, Nature 2011, 475, 183–188.
- 9
- 9aS. P. Lathrop, T. Rovis, J. Am. Chem. Soc. 2009, 131, 13628–13630;
- 9bC. M. Filloux, S. P. Lathrop, T. Rovis, Proc. Natl. Acad. Sci. USA 2010, 107, 20666–20671;
- 9cB. C. Hong, N. S. Dange, C. S. Hsu, J. H. Liao, Org. Lett. 2010, 12, 4812–4815;
- 9dH. Jiang, B. Gschwend, L. Albrecht, K. A. Jørgensen, Org. Lett. 2010, 12, 5052–5055;
- 9eD. Enders, A. Grossmann, H. Huang, G. Raabe, Eur. J. Org. Chem. 2011, 4298–4301;
- 9fB. C. Hong, N. S. Dange, C. S. Hsu, J. H. Liao, G. H. Lee, Org. Lett. 2011, 13, 1338–1341;
- 9gC. B. Jacobsen, K. L. Jensen, J. Udmark, K. A. Jørgensen, Org. Lett. 2011, 13, 4790–4793;
- 9hY. Z. Liu, J. Zhang, P. F. Xu, Y. C. Luo, J. Org. Chem. 2011, 76, 7551–7555;
- 9iK. E. Ozboya, T. Rovis, Chem. Sci. 2011, 2, 1835–1838;
- 9jZ. J. Jia, K. Jiang, Q. Q. Zhou, L. Dong, Y. C. Chen, Chem. Commun. 2013, 49, 5892–5894;
- 9kC. Ma, Z. J. Jia, J. X. Liu, Q. Q. Zhou, L. Dong, Y. C. Chen, Angew. Chem. 2013, 125, 982–985; Angew. Chem. Int. Ed. 2013, 52, 948–951.
- 10For selected examples of NHC-catalyzed cascade reactions, see:
- 10aV. Nair, S. Vellalath, M. Poonoth, E. Suresh, J. Am. Chem. Soc. 2006, 128, 8736–8737;
- 10bP. C. Chiang, J. Kaeobamrung, J. W. Bode, J. Am. Chem. Soc. 2007, 129, 3520–3521;
- 10cM. He, J. W. Bode, J. Am. Chem. Soc. 2008, 130, 418–419;
- 10dB. Cardinal-David, D. E. A. Raup, K. A. Scheidt, J. Am. Chem. Soc. 2010, 132, 5345–5347;
- 10eF. G. Sun, X. L. Huang, S. Ye, J. Org. Chem. 2010, 75, 273–276;
- 10fD. T. Cohen, B. Cardinal-David, K. A. Scheidt, Angew. Chem. 2011, 123, 1716–1720; Angew. Chem. Int. Ed. 2011, 50, 1678–1682;
- 10gX. Q. Fang, K. Jiang, C. Xing, L. Hao, Y. R. Chi, Angew. Chem. 2011, 123, 1950–1953; Angew. Chem. Int. Ed. 2011, 50, 1910–1913;
- 10hE. Sanchez-Larios, J. M. Holmes, C. L. Daschner, M. Gravel, Synthesis 2011, 1896–1904;
- 10iL. Candish, D. W. Lupton, J. Am. Chem. Soc. 2013, 135, 58–61;
- 10jL. Candish, C. M. Forsyth, D. W. Lupton, Angew. Chem. 2013, 125, 9319–9322;
10.1002/ange.201304081 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 9149–9152.
- 11For selected examples of organocatalytic activation of carboxylic acid and derivative, see:
- 11aG. S. Cortez, R. L. Tennyson, D. Romo, J. Am. Chem. Soc. 2001, 123, 7945–7946;
- 11bV. C. Purohit, A. S. Matla, D. Romo, J. Am. Chem. Soc. 2008, 130, 10478–10479;
- 11cS. J. Ryan, L. Candish, D. W. Lupton, J. Am. Chem. Soc. 2009, 131, 14176–14177;
- 11dC. A. Leverett, V. C. Purohit, D. Romo, Angew. Chem. 2010, 122, 9669–9673; Angew. Chem. Int. Ed. 2010, 49, 9479–9483;
- 11eD. Belmessieri, L. C. Morrill, C. Simal, A. M. Slawin, A. D. Smith, J. Am. Chem. Soc. 2011, 133, 2714–2720;
- 11fG. Liu, D. Romo, Angew. Chem. 2011, 123, 7679–7682; Angew. Chem. Int. Ed. 2011, 50, 7537–7540;
- 11gL. Hao, Y. Du, H. Lv, X. Chen, H. Jiang, Y. Shao, Y. R. Chi, Org. Lett. 2012, 14, 2154–2157;
- 11hL. Hao, S. Chen, J. Xu, B. Tiwari, Z. Fu, T. Li, J. Lim, Y. R. Chi, Org. Lett. 2013, 15, 4956–4959;
- 11iL. Hao, C. W. Chuen, R. Ganguly, Y. R. Chi, Synlett 2013, 1197–1200;
- 11jZ. Fu, J. Xu, T. Zhu, W. W. Y. Leong, Y. R. Chi, Nat. Chem. 2013, 5, 835–839;
- 11kS. Chen, L. Hao, Y. Zhang, B. Tiwari, Y. R. Chi, Org. Lett. 2013, 15, 5822–5825;
- 11lJ. Cheng, Z. Huang, Y. R. Chi, Angew. Chem. 2013, 125, 8754–8758; Angew. Chem. Int. Ed. 2013, 52, 8592–8596;
- 11mJ. Xu, Z. Jin, Y. R. Chi, Org. Lett. 2013, 15, 5028–5031;
- 11nL. C. Morrill, J. Douglas, T. Lebl, A. M. Z. Slawin, D. J. Fox, A. D. Smith, Chem. Sci. 2013, 4, 4146–4155;
- 11oL. Hao, X. Chen, S. Chen, K. Jiang, J. Torres, Y. R. Chi, Org. Chem. Front. 2014, 1, 148–150;
- 11pT. H. West, D. S. Daniels, A. M. Slawin, A. D. Smith, J. Am. Chem. Soc. 2014, 136, 4476–4479.
- 12P. Chauhan, D. Enders, Angew. Chem. 2014, 126, 1509–1511;
10.1002/ange.201309952 Google ScholarAngew. Chem. Int. Ed. 2014, 53, 1485–1487.
- 13
- 13aX. Q. Fang, X. K. Chen, Y. R. Chi, Org. Lett. 2011, 13, 4708–4711;
- 13bX. Q. Fang, X. K. Chen, H. Lv, Y. R. Chi, Angew. Chem. 2011, 123, 11986–11989; Angew. Chem. Int. Ed. 2011, 50, 11782–11785;
- 13cZ. Q. Fu, H. Sun, S. J. Chen, B. Tiwari, G. H. Li, Y. R. Chi, Chem. Commun. 2013, 49, 261–263.
- 14A. Bhunia, A. Patra, V. G. Puranik, A. T. Biju, Org. Lett. 2013, 15, 1756–1759.
- 15
- 15aC. M. Haskins, D. W. Knight, Tetrahedron Lett. 2004, 45, 599–601;
- 15bY. G. Liu, L. Shen, M. Prashad, J. Tibbatts, O. Repic, T. J. Blacklock, Org. Process Res. Dev. 2008, 12, 778–780;
- 15cS. P. Patel, D. H. Nadkarni, S. Murugesan, J. R. King, S. E. Velu, Synlett 2008, 2864–2868;
- 15dS. S. Michaelidou, P. A. Koutentis, Tetrahedron 2010, 66, 3016–3023;
- 15eH. Senboku, K. Nakahara, T. Fukuhara, S. Hara, Tetrahedron Lett. 2010, 51, 435–438;
- 15fS. Stokes, M. Bekkam, M. Rupp, K. T. Mead, Synlett 2012, 389–392;
- 15gV. Rajeshkumar, T. H. Lee, S. C. Chuang, Org. Lett. 2013, 15, 1468–1471.
- 16
- 16aK. Paramasivam, K. Ramasamy, P. Shanmugam, Synthesis 1977, 768–770;
- 16bR. Brettle, S. M. Shibib, J. Chem. Soc. Perkin Trans. 1 1981, 2912–2919;
- 16cS. Jaroch, H. Rehwinkel, P. Holscher, D. Sulzle, G. Burton, M. Hillmann, F. M. McDonald, H. Miklautz, Bioorg. Med. Chem. Lett. 2004, 14, 743–746;
- 16dL. E. Overman, E. A. Peterson, Heterocycles 2006, 67, 585–588.
- 17The absolute configuration of the compound 5 was confirmed by comparison of the relative structure of rac-5 and the absolute configuration of compound 3 m. CCDC 962962 (rac-5) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 18
- 18aM. Hamer, E. P. Lira, J. Heterocycl. Chem. 1972, 9, 215–218;
- 18bS. S. P. Chou, H. C. Wang, P. W. Chen, C. H. Yang, Tetrahedron 2008, 64, 5291–5297;
- 18cM. D. Mertens, M. Pietsch, G. Schnakenburg, M. Gutschow, J. Org. Chem. 2013, 78, 8966–8979;
- 18dD. G. Stark, L. C. Morrill, P. P. Yeh, A. M. Z. Slawin, T. J. C. O. Riordan, A. D. Smith, Angew. Chem. 2013, 125, 11856–11860;
10.1002/ange.201306786 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 11642–11646.
- 19
- 19aT. M. Gøgsig, A. T. Lindhardt, M. Dekhane, J. Grouleff, T. Skrydstrup, Chem. Eur. J. 2009, 15, 5950–5955;
- 19bT. M. Gogsig, A. T. Lindhardt, T. Skrydstrup, Org. Lett. 2009, 11, 4886–4888;
- 19cM. L. H. Mantel, A. T. Lindhardt, D. Lupp, T. Skrydstrup, Chem. Eur. J. 2010, 16, 5437–5442;
- 19dS. L. Fan, J. Yang, X. G. Zhang, Org. Lett. 2011, 13, 4374–4377.
- 20
- 20aG. R. Cook, L. G. Beholz, J. R. Stille, J. Org. Chem. 1994, 59, 3575–3584;
- 20bJ. G. Knight, K. Tchabanenko, Tetrahedron 2003, 59, 281–286;
- 20cS. M. Ma, B. K. Ni, Chem. Eur. J. 2004, 10, 3286–3300;
- 20dN. Kumari, Y. D. Vankar, Org. Biomol. Chem. 2009, 7, 2104–2109;
- 20eS. S. P. Chou, S. L. Chiang, G. L. Huang, B. S. Chiang, Y. C. Yu, Tetrahedron 2013, 69, 274–283.
- 21The absolute configuration of 7 was determined by comparison of the relative structure of rac-7 a and the absolute configuration of 3 m. CCDC 979557 (rac-7 a) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 22For selected examples of oxidative ring opening to a nine-membered ring, see:
- 22aD. S. Brown, M. C. Elliott, C. J. Moody, T. J. Mowlem, J. Chem. Soc. Perkin Trans. 1 1995, 1137–1144;
- 22bT. Oishi, M. Maruyama, M. Shoji, K. Maeda, N. Kumahara, S. Tanaka, M. Hirama, Tetrahedron 1999, 55, 7471–7498;
- 22cP. Wipf, W. J. Li, J. Org. Chem. 1999, 64, 4576–4577;
- 22dI. M. McDonald, D. J. Dunstone, S. B. Kalindjian, I. D. Linney, C. M. R. Low, M. J. Pether, K. I. M. Steel, M. J. Tozer, J. G. Vinter, J. Med. Chem. 2000, 43, 3518–3529;
- 22eT. J. Donohoe, A. Raoof, I. A. Linney, M. Helliwell, Org. Lett. 2001, 3, 861–864;
- 22fC. F. Pan, Z. H. Zhang, G. J. Sun, Z. Y. Wang, Org. Lett. 2004, 6, 3059–3061.