Serine-Selective Aerobic Cleavage of Peptides and a Protein Using a Water-Soluble Copper–Organoradical Conjugate†
Yohei Seki
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Search for more papers by this authorDr. Kana Tanabe
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Japan Science and Technology Agency (JST), ERATO, Kanai Life Science Catalysis Project, Bunkyo-ku, Tokyo 113-0033 (Japan)
Search for more papers by this authorDr. Daisuke Sasaki
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Japan Science and Technology Agency (JST), ERATO, Kanai Life Science Catalysis Project, Bunkyo-ku, Tokyo 113-0033 (Japan)
Search for more papers by this authorDr. Youhei Sohma
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Japan Science and Technology Agency (JST), ERATO, Kanai Life Science Catalysis Project, Bunkyo-ku, Tokyo 113-0033 (Japan)
Search for more papers by this authorCorresponding Author
Dr. Kounosuke Oisaki
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033 (Japan)Search for more papers by this authorCorresponding Author
Prof. Dr. Motomu Kanai
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Japan Science and Technology Agency (JST), ERATO, Kanai Life Science Catalysis Project, Bunkyo-ku, Tokyo 113-0033 (Japan)
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033 (Japan)Search for more papers by this authorYohei Seki
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Search for more papers by this authorDr. Kana Tanabe
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Japan Science and Technology Agency (JST), ERATO, Kanai Life Science Catalysis Project, Bunkyo-ku, Tokyo 113-0033 (Japan)
Search for more papers by this authorDr. Daisuke Sasaki
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Japan Science and Technology Agency (JST), ERATO, Kanai Life Science Catalysis Project, Bunkyo-ku, Tokyo 113-0033 (Japan)
Search for more papers by this authorDr. Youhei Sohma
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Japan Science and Technology Agency (JST), ERATO, Kanai Life Science Catalysis Project, Bunkyo-ku, Tokyo 113-0033 (Japan)
Search for more papers by this authorCorresponding Author
Dr. Kounosuke Oisaki
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033 (Japan)Search for more papers by this authorCorresponding Author
Prof. Dr. Motomu Kanai
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Japan Science and Technology Agency (JST), ERATO, Kanai Life Science Catalysis Project, Bunkyo-ku, Tokyo 113-0033 (Japan)
Graduate School of Pharmaceutical Sciences, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033 (Japan)Search for more papers by this authorThis work was supported by a Grant in-Aid for Young Scientist B and Scientific Research C from JSPS (for K.O.), and ERATO from JST (for M.K.). We thank T. Sonobe, Dr. Y. Aoi, and Dr. S. Kawashima for fruitful discussions.
Graphical Abstract
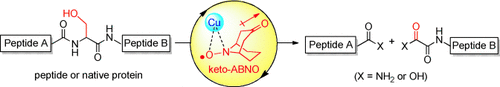
Peptides and proteins can be cleaved selectively at serine residues under mild (room temperature, near neutral pH value) aerobic conditions by a water-soluble copper–organoradical conjugate. The method is applicable to the site-selective cleavage of polypeptides that possess various functional groups, D-amino acids, or sensitive disulfide pairs. The system was also used for the site-selective cleavage of a native protein comprising more than 70 amino acid residues.
Abstract
The site-specific cleavage of peptide bonds is an important chemical modification of biologically relevant macromolecules. The reaction is not only used for routine structural determination of peptides, but is also a potential artificial modulator of protein function. Realizing the substrate scope beyond the conventional chemical or enzymatic cleavage of peptide bonds is, however, a formidable challenge. Here we report a serine-selective peptide-cleavage protocol that proceeds at room temperature and near neutral pH value, through mild aerobic oxidation promoted by a water-soluble copper–organoradical conjugate. The method is applicable to the site-selective cleavage of polypeptides that possess various functional groups. Peptides comprising D-amino acids or sensitive disulfide pairs are competent substrates. The system is extendable to the site-selective cleavage of a native protein, ubiquitin, which comprises more than 70 amino acid residues.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201402618_sm_miscellaneous_information.pdf6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Selected reviews:
- 1aJ. M. Antos, M. B. Francis, Curr. Opin. Chem. Biol. 2006, 10, 253–262;
- 1bJ. P. Pellois, T. W. Muir, Curr. Opin. Chem. Biol. 2006, 10, 487–491;
- 1cC. P. R. Hackenberger, D. Schwarzer, Angew. Chem. 2008, 120, 10182–10228;
10.1002/ange.200801313 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 10030–10074;
- 1dW. P. Heal, E. W. Tate, Org. Biomol. Chem. 2010, 8, 731–738;
- 1eY. W. Wua, R. S. Goody, J. Pept. Sci. 2010, 16, 514–523;
- 1fZ. Hao, S. Hong, S. Chen, P. R. Chen, Acc. Chem. Res. 2011, 44, 742–751;
- 1gY. Takaoka, A. Ojida, I. Hamachi, Angew. Chem. 2013, 125, 4182–4200;
10.1002/ange.201207089 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 4088–4106.
- 2Selected examples of chemical modification of native proteins:
- 2aJ. M. Antos, M. B. Francis, J. Am. Chem. Soc. 2004, 126, 10256–10257;
- 2bD. Bang, B. Pentelute, S. B. H. Kent, Angew. Chem. 2006, 118, 4089–4092;
10.1002/ange.200600702 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 3985–3988;
- 2cG. J. L. Bernardes, J. M. Chalker, J. C. Errey, B. G. Davis, J. Am. Chem. Soc. 2008, 130, 5052–5053;
- 2dS. Tsukiji, M. Miyagawa, Y. Takaoka, T. Tamura, I. Hamachi, Nat. Chem. Biol. 2009, 5, 341–343;
- 2eH. Ban, J. Gavrilyuk, C. F. Barbas III, J. Am. Chem. Soc. 2010, 132, 1523–1525;
- 2fB. V. Popp, Z. T. Ball, J. Am. Chem. Soc. 2010, 132, 6660–6662;
- 2gS. Sato, H. Nakamura, Angew. Chem. 2013, 125, 8843–8846; Angew. Chem. Int. Ed. 2013, 52, 8681–8684.
- 3S. L. Schreiber, Nat. Chem. Biol. 2005, 1, 64–66.
- 4C. J. O’Conner, L. Laraia, D. R. Spring, Chem. Soc. Rev. 2011, 40, 4332–4345.
- 5
- 5aM. Leisola, O. Turunen, Appl. Microbiol. Biotechnol. 2007, 75, 1225–1232;
- 5bB. A. Smith, M. H. Hecht, Curr. Opin. Chem. Biol. 2011, 15, 421–426.
- 6
- 6aR. Aebersold, M. Mann, Nature 2003, 422, 198–207;
- 6bR. S. Morrison, Y. Kinoshita, M. D. Johnson, T. P. Conrads, Adv. Protein Chem. 2003, 65, 1–23.
- 7
- 7aM. A. Gallop, R. W. Barrett, W. J. Dower, S. P. A. Fodor, E. M. Gordon, J. Med. Chem. 1994, 37, 1233–1251;
- 7bA. Cruz-Migoni, N. Fuentes-Fernandez, T. H. Rabbitts, Med. Chem. Commun. 2013, 4, 1218–1221.
- 8Selected examples of the chemical cleavage of peptides targeting native amino acid residues:
- 8aE. Gross, B. Witkop, J. Biol. Chem. 1962, 237, 1856–1860;
- 8bA. Patchornik, M. Sokolovsky, J. Am. Chem. Soc. 1964, 86, 1206–1212;
- 8cD. Ranganathan, S. Saini, J. Am. Chem. Soc. 1991, 113, 1042–1044;
- 8dD. Ranganathan, N. K. Vaish, K. Shah, J. Am. Chem. Soc. 1994, 116, 6545–6557;
- 8eE. J. Corey, L. F. Haefele, J. Am. Chem. Soc. 1959, 81, 2225–2228;
- 8fG. L. Schmir, L. A. Cohen, B. Witkop, J. Am. Chem. Soc. 1959, 81, 2228–2233;
- 8gN. M. Alexander, J. Biol. Chem. 1974, 249, 1946–1952;
- 8hA. R. Ekkati, J. J. Kodanko, J. Am. Chem. Soc. 2007, 129, 12390–12391;
- 8iA. I. Abouelatta, A. A. Campanali, A. R. Ekkati, M. Shamoun, S. Kalapugama, J. J. Kodanko, Inorg. Chem. 2009, 48, 7729–7739;
- 8jS. Murahashi, A. Mitani, K. Kitao, Tetrahedron Lett. 2000, 41, 10245–10249;
- 8kK. Tanabe, A. Taniguchi, T. Matsumoto, K. Oisaki, Y. Sohma, M. Kanai, Chem. Sci. 2014, DOI: .
- 9
- 9aT. Y. Lee, J. Suh, Chem. Soc. Rev. 2009, 38, 1949–1957;
- 9bJ. Prakash, J. J. Kodanko, Curr. Opin. Chem. Biol. 2013, 17, 197–203.
- 10A. Taniguchi, D. Sasaki, A. Shiohara, T. Iwatsubo, T. Tomita, Y. Sohma, M. Kanai, Angew. Chem. 2014, 126, 1406–1409; Angew. Chem. Int. Ed. 2014, 53, 1382–1385.
- 11
- 11aJ. Arnau, C. Lauritzen, G. E. Petersen, Protein Expression Purif. 2006, 48, 1–13;
- 11b New Methods in Peptide Mapping for the Characterization of Proteins (Ed.: ), CRC, Boca Raton, FL, 1996.
- 12Selected examples of metal-catalyzed hydrolytic cleavage of peptides:
- 12aL. Zhu, N. M. Kostić, J. Am. Chem. Soc. 1993, 115, 4566–4570;
- 12bL. Zhu, L. Qin, T. N. Parac, N. M. Kostić, J. Am. Chem. Soc. 1994, 116, 5218–5224;
- 12cN. V. Kaminskaia, T. W. Johnson, N. M. Kostić, J. Am. Chem. Soc. 1999, 121, 8663–8664;
- 12dN. M. Milović, N. M. Kostić, J. Am. Chem. Soc. 2002, 124, 4759–4769;
- 12eA. Kréżel, E. Kopera, A. M. Protas, J. Poznański, A. Wysłouch-Cieszyńska, W. Bal, J. Am. Chem. Soc. 2010, 132, 3355–3366;
- 12fD. Hoyer, H. Cho, P. G. Schultz, J. Am. Chem. Soc. 1990, 112, 3249–3250;
- 12gE. L. Hegg, J. N. Burstyn, J. Am. Chem. Soc. 1995, 117, 7015–7016;
- 12hM. A. Smith, M. Easton, P. Everett, G. Lewis, M. Payne, V. Riveros-Moreno, G. Allen, Int. J. Peptide Protein Res. 1996, 48, 48–55;
- 12iT. Takarada, M. Yashiro, M. Komiyama, Chem. Eur. J. 2000, 6, 3906–3913;
10.1002/1521-3765(20001103)6:21<3906::AID-CHEM3906>3.0.CO;2-J CAS PubMed Web of Science® Google Scholar
- 12jK. Stroobants, E. Moelants, H. G. T. Ly, P. Proost, K. Bartik, T. N. Parac-Vogt, Chem. Eur. J. 2013, 19, 2848–2858;
- 12kJ. P. Collman, D. A. Buckingham, J. Am. Chem. Soc. 1963, 85, 3039–3040;
- 12lA. Schepartz, R. Breslow, J. Am. Chem. Soc. 1987, 109, 1814–1826;
- 12mA. Schepartz, B. Cuenoud, J. Am. Chem. Soc. 1990, 112, 3247–3249;
- 12nN. Ettner, W. Hillen, J. Am. Chem. Soc. 1993, 115, 2546–2548.
- 13A. Radzicka, R. Wolfenden, J. Am. Chem. Soc. 1996, 118, 6105–6109.
- 14Y. Kita, Y. Nishii, T. Higuchi, K. Mashima, Angew. Chem. 2012, 124, 5821–5824; Angew. Chem. Int. Ed. 2012, 51, 5723–5726.
- 15Aerobic oxidation of alcohols by Cu or Fe/N-oxyl radical catalysis:
- 15aM. F. Semmelhack; C. R. Schmid, D. A. Cortés, C. S. Chou, J. Am. Chem. Soc. 1984, 106, 3374–3376; C. R. Schmid, D. A. Cortés, C. S. Chou, J. Am. Chem. Soc. 1984, 106, 3374–3376;
- 15bN. Wang, R. Liu, J. Chen, X. Liang, Chem. Commun. 2005, 5322–5324;
- 15cM. H. Jessica, S. S. Stahl, J. Am. Chem. Soc. 2011, 133, 16901–16910;
- 15dJ. E. Steves, S. S. Stahl, J. Am. Chem. Soc. 2013, 135, 15742–15745, and references therein.
- 16Aerobic oxidation of amines by Cu/N-oxyl radical catalysis:
- 16aZ. Hu, F. M. Kerton, Org. Biomol. Chem. 2012, 10, 1618–1624;
- 16bT. Sonobe, K. Oisaki, M. Kanai, Chem. Sci. 2012, 3, 3249–3255;
- 16cB. Huang, H. Tian, S. Lin, M. Xie, X. Yu, Q. Xu, Tetrahedron Lett. 2013, 54, 2861–2864;
- 16dJ. Kim, S. S. Stahl, ACS Catal. 2013, 3, 1652–1656.
- 17Selected reviews:
- 17aI. W. C. E. Arends, Y.-X. Li, E. Aussan, R. A. Sheldon, J. Mol. Catal. A 2006, 251, 200–214;
- 17bJ. M. Bobbitt, C. Brückner, N. Merbouh, Org. React. 2009, 74, 103–424;
- 17cL. Tebben, A. Studer, Angew. Chem. 2011, 123, 5138–5174;
10.1002/ange.201002547 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 5034–5068;
- 17dA. E. Wendlandt, A. M. Suess, S. S. Stahl, Angew. Chem. 2011, 123, 11256–11283;
10.1002/ange.201103945 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 11062–11087;
- 17eS. E. Allen, R. R. Walvoord, R. Padilla-Salinas, M. C. Kozlowski, Chem. Rev. 2013, 113, 6234–6458;
- 17fY. Iwabuchi, Chem. Pharm. Bull. 2013, 61, 1197–1213.
- 18J. J. Gorman, T. P. Wallis, J. J. Pitt, Mass Spectrom. Rev. 2002, 21, 183–216.
- 19S. Vijay-Kumar, C. E. Bugg, W. J. Cook, J. Mol. Biol. 1987, 194, 531–544.
- 20Recent mechanistic discussions of catalytic oxidation of alcohols by Cu/TEMPO/O2 system:
- 20aL. Cheng, J. Wang, M. Wang, Z. Wu, Inorg. Chem. 2010, 49, 9392–9399;
- 20bP. Belanzoni, C. Michel, E. J. Baerends, Inorg. Chem. 2011, 50, 11896–11904;
- 20cJ. M. Hoover, B. L. Ryland, S. S. Stahl, J. Am. Chem. Soc. 2013, 135, 2357–2367;
- 20dJ. M. Hoover, B. L. Ryland, S. S. Stahl, ACS Catal. 2013, 3, 2599–2605. For amine oxidation, see ref. [16b].
- 21Discussions of [1e+1e] oxidation mechanism in Cu-peroxide catalysis:
- 21aE. Boess, D. Sureshkumar, A. Sud, C. Wirtz, C. Fares, M. Klussmann, J. Am. Chem. Soc. 2011, 133, 8106–8109;
- 21bS. Hashizume, K. Oisaki, M. Kanai, Chem. Asian J. 2012, 7, 2600–2606;
- 21cC. Zhang, C. Tang, N. Jiao, Chem. Soc. Rev. 2012, 41, 3464–3484.
- 22Acceleration effect of NOx additive (acid + NaNO2) in the oxidation catalyzed by N-oxyl radical:
- 22aR. Liu, X. Liang, C. Dong, X. Hu, J. Am. Chem. Soc. 2004, 126, 4112–4113;
- 22bY. Xie, W. Mo, D. Xu, Z. Shen, N. Sun, B. Hu, X. Hu, J. Org. Chem. 2007, 72, 4288–4291;
- 22cX. Wang, R. Liu, Y. Jin, X. Liang, Chem. Eur. J. 2008, 14, 2679–2685;
- 22dA. Rahimi, A. Azarpira, H. Kim, J. Ralph, S. S. Stahl, J. Am. Chem. Soc. 2013, 135, 6415–6418, and references therein.
- 23We could not detect putative intermediates B or B′ because the oxidation from A to C was very fast. Although a detailed reaction mechanism is still controversial, there are several reports documenting that easily enolizable α-(acylamino)aldehydes were rapidly converted to oxalimides through CC bond cleavage through B and B′, even under mild oxidative conditions. For such precedents, see:
- 23aW. von E. Doering, R. M. Haines, J. Am. Chem. Soc. 1954, 76, 482–486;
- 23bP. Meffre, L. Gauzy, E. Branquet, P. Durand, F. Le Goffic, Tetrahedron 1996, 52, 11215–11238, and references therein;
- 23cG. Cabarrocas, M. Ventura, M. Maestro, J. Mahía, J. M. Villalgordo, Tetrahedron: Asymmetry 2001, 12, 1851–1863;
- 23dM. García-Valverde, R. Pedrosa, M. Vicente, Synlett 2002, 2092–2094;
- 23eL. M. Sayre, S.-J. Jin, J. Org. Chem. 1984, 49, 3498–3503;
- 23fS.-J. Jin, P. K. Arora, L. M. Sayre, J. Org. Chem. 1990, 55, 3011–3018.