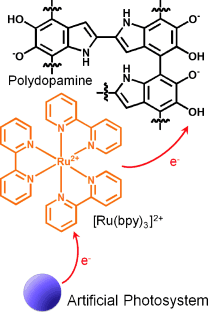
Polydopamine as a Biomimetic Electron Gate for Artificial Photosynthesis†
This study was supported by grants from the National Research Foundation (NRF) through the National Leading Research Laboratory (NRF-2013R1A2A1A05005468), the Intelligent Synthetic Biology Center of Global Frontier R&D Project (2011-0031957), and the Converging Research Center (2013K000236), Republic of Korea.
Graphical Abstract
Nature as role model: Comparable to quinones that extract electrons from chlorophyll in the natural photosystem II, polydopamine (PDA) accelerates proton-coupled electron transfer and enables efficient charge separation of [Ru(bpy)3]2+. The introduction of PDA as an electron gate as well as a versatile adhesive significantly increases the efficiency of photochemical water oxidation.
Abstract
We report on the capability of polydopamine (PDA), a mimic of mussel adhesion proteins, as an electron gate as well as a versatile adhesive for mimicking natural photosynthesis. This work demonstrates that PDA accelerates the rate of photoinduced electron transfer from light-harvesting molecules through two-electron and two-proton redox-coupling mechanism. The introduction of PDA as a charge separator significantly increased the efficiency of photochemical water oxidation. Furthermore, simple incorporation of PDA ad-layer on the surface of conducting materials, such as carbon nanotubes, facilitated fast charge separation and oxygen evolution through the synergistic effect of PDA-mediated proton-coupled electron transfer and the high conductivity of the substrate. Our work shows that PDA is an excellent electron acceptor as well as a versatile adhesive; thus, PDA constitutes a new electron gate for harvesting photoinduced electrons and designing artificial photosynthetic systems.