Highly Reactive Nonheme Iron(III) Iodosylarene Complexes in Alkane Hydroxylation and Sulfoxidation Reactions†
Dr. Seungwoo Hong
Department of Chemistry and Nano Science, Ewha Womans University, Seoul 120–750 (Korea)
These authors contributed equally to this work.
Search for more papers by this authorDr. Bin Wang
Department of Chemistry and Nano Science, Ewha Womans University, Seoul 120–750 (Korea)
These authors contributed equally to this work.
Search for more papers by this authorDr. Mi Sook Seo
Department of Chemistry and Nano Science, Ewha Womans University, Seoul 120–750 (Korea)
Search for more papers by this authorDr. Yong-Min Lee
Department of Chemistry and Nano Science, Ewha Womans University, Seoul 120–750 (Korea)
Search for more papers by this authorMyoung Jin Kim
Department of Chemistry and Nano Science, Ewha Womans University, Seoul 120–750 (Korea)
Search for more papers by this authorProf. Dr. Hyung Rok Kim
Research Center for Green Catalysis, Korea Research Institute of Chemical Technology, Daejeon 305-600 (Korea)
Search for more papers by this authorProf. Dr. Takashi Ogura
Picobiology Institute, Graduate School of Life Science, University of Hyogo, Koto 3-2-1, Kamigori-cho, Ako-gun, Hyogo 678-1297 (Japan)
Search for more papers by this authorDr. Ricardo Garcia-Serres
University of Grenoble Alpes, LCBM, 38054 Grenoble (France)
CEA, DSV, iRTSV, LCBM, PMB, 38054 Grenoble (France)
Search for more papers by this authorDr. Martin Clémancey
CNRS UMR 5249, LCBM, 38054 Grenoble (France)
CEA, DSV, iRTSV, LCBM, PMB, 38054 Grenoble (France)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jean-Marc Latour
CEA, DSV, iRTSV, LCBM, PMB, 38054 Grenoble (France)
Jean-Marc Latour, CEA, DSV, iRTSV, LCBM, PMB, 38054 Grenoble (France)
Wonwoo Nam, Department of Chemistry and Nano Science, Ewha Womans University, Seoul 120–750 (Korea)
Search for more papers by this authorCorresponding Author
Prof. Dr. Wonwoo Nam
Department of Chemistry and Nano Science, Ewha Womans University, Seoul 120–750 (Korea)
Jean-Marc Latour, CEA, DSV, iRTSV, LCBM, PMB, 38054 Grenoble (France)
Wonwoo Nam, Department of Chemistry and Nano Science, Ewha Womans University, Seoul 120–750 (Korea)
Search for more papers by this authorDr. Seungwoo Hong
Department of Chemistry and Nano Science, Ewha Womans University, Seoul 120–750 (Korea)
These authors contributed equally to this work.
Search for more papers by this authorDr. Bin Wang
Department of Chemistry and Nano Science, Ewha Womans University, Seoul 120–750 (Korea)
These authors contributed equally to this work.
Search for more papers by this authorDr. Mi Sook Seo
Department of Chemistry and Nano Science, Ewha Womans University, Seoul 120–750 (Korea)
Search for more papers by this authorDr. Yong-Min Lee
Department of Chemistry and Nano Science, Ewha Womans University, Seoul 120–750 (Korea)
Search for more papers by this authorMyoung Jin Kim
Department of Chemistry and Nano Science, Ewha Womans University, Seoul 120–750 (Korea)
Search for more papers by this authorProf. Dr. Hyung Rok Kim
Research Center for Green Catalysis, Korea Research Institute of Chemical Technology, Daejeon 305-600 (Korea)
Search for more papers by this authorProf. Dr. Takashi Ogura
Picobiology Institute, Graduate School of Life Science, University of Hyogo, Koto 3-2-1, Kamigori-cho, Ako-gun, Hyogo 678-1297 (Japan)
Search for more papers by this authorDr. Ricardo Garcia-Serres
University of Grenoble Alpes, LCBM, 38054 Grenoble (France)
CEA, DSV, iRTSV, LCBM, PMB, 38054 Grenoble (France)
Search for more papers by this authorDr. Martin Clémancey
CNRS UMR 5249, LCBM, 38054 Grenoble (France)
CEA, DSV, iRTSV, LCBM, PMB, 38054 Grenoble (France)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jean-Marc Latour
CEA, DSV, iRTSV, LCBM, PMB, 38054 Grenoble (France)
Jean-Marc Latour, CEA, DSV, iRTSV, LCBM, PMB, 38054 Grenoble (France)
Wonwoo Nam, Department of Chemistry and Nano Science, Ewha Womans University, Seoul 120–750 (Korea)
Search for more papers by this authorCorresponding Author
Prof. Dr. Wonwoo Nam
Department of Chemistry and Nano Science, Ewha Womans University, Seoul 120–750 (Korea)
Jean-Marc Latour, CEA, DSV, iRTSV, LCBM, PMB, 38054 Grenoble (France)
Wonwoo Nam, Department of Chemistry and Nano Science, Ewha Womans University, Seoul 120–750 (Korea)
Search for more papers by this authorWe gratefully acknowledge research support of this work by the NRF of Korea through CRI (NRF-2012R1A3A2048842 to W.N.), GRL (NRF-2010-00353 to W.N.), and Basic Research Program (2010-0002558 to M.S.S.). J.M.L. acknowledges the support of Labex ARCANE (ANR-11-LABX-0003-01). T.O. acknowledges support by Grant-in-Aid for Scientific Research on Innovative Areas (No. 25109540) from MEXT (Japan).
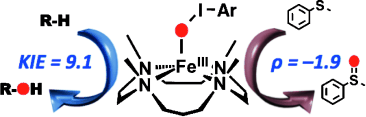
Graphical Abstract
An iron boost: High-spin iron(III) iodosylarene complexes bearing an N-methylated cyclam ligand are prepared. The nonheme high-spin iron(III) iodosylarene intermediates are highly reactive oxidants capable of activating strong CH bonds of alkanes. The electrophilic character of the iron(III) iodosylarene complexes is demonstrated in sulfoxidation reactions.
Abstract
High-spin iron(III) iodosylarene complexes bearing an N-methylated cyclam ligand are synthesized and characterized using various spectroscopic methods. The nonheme high-spin iron(III) iodosylarene intermediates are highly reactive oxidants capable of activating strong CH bonds of alkanes; the reactivity of the iron(III) iodosylarene intermediates is much greater than that of the corresponding iron(IV) oxo complex. The electrophilic character of the iron(III) iodosylarene complexes is demonstrated in sulfoxidation reactions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201402537_sm_miscellaneous_information.pdf836.2 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aW. Nam, Acc. Chem. Res. 2007, 40, 465, and references therein;
- 1bM. M. Abu-Omar, A. Loaiza, N. Hontzeas, Chem. Rev. 2005, 105, 2227;
- 1cC. Krebs, D. G. Fujimori, C. T. Walsh, J. M. Bollinger, Jr., Acc. Chem. Res. 2007, 40, 484;
- 1dP. C. A. Bruijnincx, G. van Koten, R. J. M. Klein Gebbink, Chem. Soc. Rev. 2008, 37, 2716;
- 1eJ. Rittle, M. T. Green, Science 2010, 330, 933.
- 2
- 2aW. Nam, Acc. Chem. Res. 2007, 40, 522;
- 2bA. Gunay, K. H. Theopold, Chem. Rev. 2010, 110, 1060;
- 2cA. S. Borovik, Chem. Soc. Rev. 2011, 40, 1870;
- 2dJ. Hohenberger, K. Ray, K. Meyer, Nat. Commun. 2012, 3, 720;
- 2eS. P. de Visser, J.-U. Rohde, Y.-M. Lee, J. Cho, W. Nam, Coord. Chem. Rev. 2013, 257, 381;
- 2fA. R. Mcdonalde, L. Que, Jr., Coord. Chem. Rev. 2013, 257, 414;
- 2g W. Nam, Y.-M. Lee, S. Fukuzumi, Acc. Chem. Res. 2014, 47, 1146.
- 3
- 3aY.-M. Lee, S. Hong, Y. Morimoto, W. Shin, S. Fukuzumi, W. Nam, J. Am. Chem. Soc. 2010, 132, 10668;
- 3bA. Mukherjee, M. A. Cranswick, M. Chakrabarti, T. K. Paine, K. Fujisawa, E. Münck, L. Que, Jr., Inorg. Chem. 2010, 49, 3618.
- 4
- 4aJ. Cho, S. Jeon, S. A. Wilson, L. V. Liu, E. A. Kang, J. J. Braymer, M. H. Lim, B. Hedman, K. O. Hodgson, J. S. Valentine, E. I. Solomon, W. Nam, Nature 2011, 478, 502;
- 4bL. V. Liu, S. Hong, J. Cho, W. Nam, E. I. Solomon, J. Am. Chem. Soc. 2013, 135, 3286.
- 5
- 5aC. Wang, T. Kurahashi, H. Fujii, Angew. Chem. 2012, 124, 7929; Angew. Chem. Int. Ed. 2012, 51, 7809;
- 5bZ. Cong, S. Yanagisawa, T. Kurahashi, T. Ogura, S. Nakashima, H. Fujii, J. Am. Chem. Soc. 2012, 134, 20617;
- 5cC. Wang, T. Kurahashi, K. Inomata, M. Hada, H. Fujii, Inorg. Chem. 2013, 52, 9557.
- 6A. Lennartson, C. J. McKenzie, Angew. Chem. 2012, 124, 6871;
10.1002/ange.201202487 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 6767.
- 7M. Guo, H. Dong, J. Li, B. Cheng, Y.-q. Huang, Y.-q. Feng, A. Lei, Nat. Commun. 2012, 3, 1190.
- 8
- 8aS. Shaik, H. Hirao, D. Kumar, Nat. Prod. Rep. 2007, 24, 533;
- 8bW. Nam, Y. O. Ryu, W. J. Song, J. Biol. Inorg. Chem. 2004, 9, 654;
- 8cM. Newcomb, P. F. Hollenberg, M. J. Coon, Arch. Biochem. Biophys. 2003, 409, 72.
- 9
- 9aM. Newcomb, P. H. Toy, Acc. Chem. Res. 2000, 33, 449;
- 9bW. Nam, M. H. Lim, H. J. Lee, C. Kim, J. Am. Chem. Soc. 2000, 122, 6641;
- 9cJ. P. Collman, A. S. Chien, T. A. Eberspacher, J. I. Brauman, J. Am. Chem. Soc. 2000, 122, 11098;
- 9dS. H. Wang, B. S. Mandimutsira, R. Todd, B. Ramdhanie, J. P. Fox, D. P. Goldberg, J. Am. Chem. Soc. 2004, 126, 18;
- 9eK. P. Bryliakov, E. P. Talsi, Angew. Chem. 2004, 116, 5340;
10.1002/ange.200460108 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5228;
- 9fW. J. Song, M. S. Seo, S. D. George, T. Ohta, R. Song, M.-J. Kang, T. Tosha, T. Kitagawa, E. I. Solomon, W. Nam, J. Am. Chem. Soc. 2007, 129, 1268;
- 9gP. Leeladee, D. P. Goldberg, Inorg. Chem. 2010, 49, 3083.
- 10
- 10aS. Hong, H. So, H. Yoon, K.-B. Cho, Y.-M. Lee, S. Fukuzumi, W. Nam, Dalton Trans. 2013, 42, 7842;
- 10bS. Hong, Y.-M. Lee, K.-B. Cho, M. S. Seo, D. Song, J. Yoon, R. Garcia-Serres, M. Clémancey, T. Ogura, W. Shin, J.-M. Latour, W. Nam, Chem. Sci. 2014, 5, 156.
- 11Broad 16O/18O isotope-sensitive IR bands for IOMn bonds were reported at ca. 600 cm−1 in MnIV–iodosylarene adducts,[5a,b] whereas a rRaman band at 780 cm−1 was assigned to an OCl stretching mode in (porphyrin)FeIII–OCl species.[5b]
- 12C. E. MacBeth, R. Gupta, K. R. Mitchell-Koch, V. G. Young, Jr., G. H. Lushington, W. H. Thompson, M. P. Hendrich, A. S. Borovik, J. Am. Chem. Soc. 2004, 126, 2556.
- 13W. Nam, J. S. Valentine, J. Am. Chem. Soc. 1993, 115, 1772.
- 14We cannot exclude the possibility that the oxygen exchange between iron(III) iodosylarene complexes and H218O may occur after the iodosylarene in the iron(III) iodosylarene complexes dissociates first and exchanges with H218O. Then, the 18O-labeled iodosylarenes are rebound to an iron(III) species to give the 18O-labeled iron(III) iodosylarene complexes.
- 15
- 15aJ. Arias, C. R. Newlands, M. M. Abu-Omar, Inorg. Chem. 2001, 40, 2185;
- 15bM. Taki, S. Itoh, S. Fukuzumi, J. Am. Chem. Soc. 2002, 124, 998;
- 15cL. D. McPherson, M. Drees, S. I. Khan, T. Strassner, M. M. Abu-Omar, Inorg. Chem. 2004, 43, 4036.
- 16
- 16aJ. Cho, J. Woo, W. Nam, J. Am. Chem. Soc. 2010, 132, 5958;
- 16bY. M. Kim, K.-B. Cho, J. Cho, B. Wang, C. Li, S. Shaik, W. Nam, J. Am. Chem. Soc. 2013, 135, 8838.
- 17
- 17aW. Nam, S. K. Choi, M. H. Lim, J.-U. Rohde, I. Kim, J. Kim, C. Kim, L. Que, Jr., Angew. Chem. 2003, 115, 113;
10.1002/ange.200390004 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 109;
- 17bW. J. Song, Y. J. Sun, S. K. Choi, W. Nam, Chem. Eur. J. 2006, 12, 130.