Oxygen-Promoted CH Bond Activation at Palladium†
Dr. Margaret L. Scheuermann
Department of Chemistry, University of Washington, Box 351700, Seattle, WA 98195 (USA)
Search for more papers by this authorDavid W. Boyce
Department of Chemistry and Center for Metals in Biocatalysis, University of Minnesota, 207 Pleasant Street SE, Minneapolis, MN 55455 (USA)
Search for more papers by this authorProf. Kyle A. Grice
Department of Chemistry, University of Washington, Box 351700, Seattle, WA 98195 (USA)
Present address: Department of Chemistry, DePaul University, 1110 West Belden Avenue, Chicago, IL 60614 (USA)
Search for more papers by this authorProf. Werner Kaminsky
Department of Chemistry, University of Washington, Box 351700, Seattle, WA 98195 (USA)
Search for more papers by this authorProf. Stefan Stoll
Department of Chemistry, University of Washington, Box 351700, Seattle, WA 98195 (USA)
Search for more papers by this authorProf. William B. Tolman
Department of Chemistry and Center for Metals in Biocatalysis, University of Minnesota, 207 Pleasant Street SE, Minneapolis, MN 55455 (USA)
Search for more papers by this authorProf. Ole Swang
inGAP Centre for Research-Based Innovation, Department of Chemistry, University of Oslo, P.O. Box 1033, Blindern, 0315 Oslo (Norway)
SINTEF Materials and Chemistry, P.O. Box 124, Blindern, 0314 Oslo (Norway)
Search for more papers by this authorCorresponding Author
Prof. Karen I. Goldberg
Department of Chemistry, University of Washington, Box 351700, Seattle, WA 98195 (USA)
Department of Chemistry, University of Washington, Box 351700, Seattle, WA 98195 (USA)Search for more papers by this authorDr. Margaret L. Scheuermann
Department of Chemistry, University of Washington, Box 351700, Seattle, WA 98195 (USA)
Search for more papers by this authorDavid W. Boyce
Department of Chemistry and Center for Metals in Biocatalysis, University of Minnesota, 207 Pleasant Street SE, Minneapolis, MN 55455 (USA)
Search for more papers by this authorProf. Kyle A. Grice
Department of Chemistry, University of Washington, Box 351700, Seattle, WA 98195 (USA)
Present address: Department of Chemistry, DePaul University, 1110 West Belden Avenue, Chicago, IL 60614 (USA)
Search for more papers by this authorProf. Werner Kaminsky
Department of Chemistry, University of Washington, Box 351700, Seattle, WA 98195 (USA)
Search for more papers by this authorProf. Stefan Stoll
Department of Chemistry, University of Washington, Box 351700, Seattle, WA 98195 (USA)
Search for more papers by this authorProf. William B. Tolman
Department of Chemistry and Center for Metals in Biocatalysis, University of Minnesota, 207 Pleasant Street SE, Minneapolis, MN 55455 (USA)
Search for more papers by this authorProf. Ole Swang
inGAP Centre for Research-Based Innovation, Department of Chemistry, University of Oslo, P.O. Box 1033, Blindern, 0315 Oslo (Norway)
SINTEF Materials and Chemistry, P.O. Box 124, Blindern, 0314 Oslo (Norway)
Search for more papers by this authorCorresponding Author
Prof. Karen I. Goldberg
Department of Chemistry, University of Washington, Box 351700, Seattle, WA 98195 (USA)
Department of Chemistry, University of Washington, Box 351700, Seattle, WA 98195 (USA)Search for more papers by this authorThis work was supported by the National Science Foundation under Grant Nos. CHE-1012045 and DGE-0718124. Computational studies were enabled by the Research Council of Norway through its support of the NOTUR program for computing resources and the CoE Centre for Theoretical and Computational Chemistry (CTCC), Grant No. 179568V30. We thank Professors Christopher J. Cramer (University of Minnesota) and Mats Tilset (University of Oslo) for valuable discussions.
Graphical Abstract
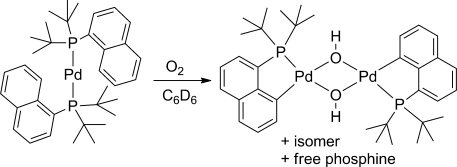
Oxygen leads the way: [Pd(P(Ar)(tBu)2)2] (Ar=naphthyl) undergoes a reaction with molecular oxygen in which CH and OO bonds are cleaved. Observation of the reaction at low temperature suggests the initial formation of a superoxo complex, which then generates a peroxo complex prior to the CH activation step. The transition state for an energetically viable CH activation across a Pdperoxo bond was located computationally.
Abstract
[Pd(P(Ar)(tBu)2)2] (1, Ar=naphthyl) reacts with molecular oxygen to form PdII hydroxide dimers in which the naphthyl ring is cyclometalated and one equivalent of phosphine per palladium atom is released. This reaction involves the cleavage of both CH and OO bonds, two transformations central to catalytic aerobic oxidizations of hydrocarbons. Observations at low temperature suggest the initial formation of a superoxo complex, which then generates a peroxo complex prior to the CH activation step. A transition state for energetically viable CH activation across a Pdperoxo bond was located computationally.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201402484_sm_miscellaneous_information.pdf1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. S. Stahl, Science 2005, 309, 1824.
- 2F. Cavani, J. H. Teles, ChemSusChem 2009, 2, 508.
- 3
- 3aL. Boisvert, K. I. Goldberg, Acc. Chem. Res. 2012, 45, 899;
- 3bA. N. Campbell, S. S. Stahl, Acc. Chem. Res. 2012, 45, 851;
- 3cZ. Shi, C. Zhang, C. Tang, N. Jiao, Chem. Soc. Rev. 2012, 41, 3381;
- 3dM. J. Schultz, M. S. Sigman, Tetrahedron 2006, 62, 8227;
- 3eT. Punniyamurthy, S. Velusamy, J. Iqbal, Chem. Rev. 2005, 105, 2329.
- 4
- 4aM. S. Sigman, D. R. Jensen, Acc. Chem. Res. 2006, 39, 221;
- 4bK. M. Gligorich, M. S. Sigman, Angew. Chem. 2006, 118, 6764;
10.1002/ange.200602138 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 6612.
- 5
- 5aW. Wu, H. Jiang, Acc. Chem. Res. 2012, 45, 1736;
- 5bJ. M. Takacs, X. T. Jiang, Curr. Org. Chem. 2003, 7, 369;
- 5cJ. Smidt, W. Hafner, R. Jira, R. Sieber, J. Sedlmeier, A. Sabel, Angew. Chem. 1962, 74, 93; Angew. Chem. Int. Ed. Engl. 1962, 1, 80.
- 6
- 6aT. Jintoku, K. Nishimura, K. Takaki, Y. Fujiwara, Chem. Lett. 1991, 193;
- 6bT. Jintoku, K. Nishimura, K. Takaki, Y. Fujiwara, Chem. Lett. 1990, 1687;
- 6cT. Jintoku, K. Takaki, Y. Fujiwara, Y. Fuchita, K. Hiraki, Bull. Chem. Soc. Jpn. 1990, 63, 438;
- 6dT. Jintoku, H. Taniguchi, Y. Fujiwara, Chem. Lett. 1987, 1865.
- 7S. Yamada, S. Sakaguchi, Y. Ishii, J. Mol. Catal. A 2007, 262, 48.
- 8Y.-H. Zhang, J.-Q. Yu, J. Am. Chem. Soc. 2009, 131, 14654.
- 9G. J. Chuang, W. Wang, E. Lee, T. Ritter, J. Am. Chem. Soc. 2011, 133, 1760.
- 10Y. Yan, P. Feng, Q.-Z. Zheng, Y.-F. Liang, J.-F. Lu, Y. Cui, N. Jiao, Angew. Chem. 2013, 125, 5939; Angew. Chem. Int. Ed. 2013, 52, 5827.
- 11
- 11aH. Li, B.-J. Li, Z.-J. Shi, Catal. Sci. Technol. 2011, 1, 191;
- 11bT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147.
- 12V. Durà-Vilà, D. M. P. Mingos, R. Vilar, A. J. P. White, D. J. Williams, Chem. Commun. 2000, 1525.
- 13W. de Graaf, J. Boersma, W. J. J. Smeets, A. L. Spek, G. van Koten, Organometallics 1989, 8, 2907.
- 14A. Karaçar, M. Freytag, H. Thönnessen, P. G. Jones, R. Bartsch, R. Schmutzler, J. Organomet. Chem. 2002, 643–644, 68.
- 15Persistence of Vision Ray-Tracer (POV-Ray), available at http://www.povray.org/.
- 16CCDC 983922 (1) and 983923 (2 a) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 17J. P. Perdew, Phys. Rev. B 1986, 33, 8822.
- 18E. van Lenthe, E. J. Baerends, J. Comput. Chem. 2003, 24, 1142.
- 19M. Brookhart, M. L. H. Green, G. Parkin, Proc. Natl. Acad. Sci. USA 2007, 104, 6908.
- 20V. V. Grushin, H. Alper, Organometallics 1993, 12, 1890.
- 21See the Supporting Information for full details.
- 22For examples see:
- 22aT. Yoshida, K. Tatsumi, M. Matsumoto, K. Nakatsu, A. Nakamura, T. Fueno, S. Otsuka, Nouv. J. Chim. 1979, 3, 761;
- 22bS. Otsuka, T. Yoshida, M. Matsumoto, K. Nakatsu, J. Am. Chem. Soc. 1976, 98, 5850;
- 22cM. Matsumoto, H. Yoshioka, K. Nakatsu, T. Yoshida, S. Otsuka, J. Am. Chem. Soc. 1974, 96, 3322.
- 23For example see: M. M. Konnick, I. A. Guzei, S. S. Stahl, J. Am. Chem. Soc. 2004, 126, 10212.
- 24X. Cai, S. Majumdar, G. C. Fortman, C. S. J. Cazin, A. M. Z. Slawin, C. Lhermitte, R. Prabhakar, M. E. Germain, T. Palluccio, S. P. Nolan, E. V. Rybak-Akimova, M. Temprado, B. Captain, C. D. Hoff, J. Am. Chem. Soc. 2011, 133, 1290.
- 25At room temperature, the best yields of 2 a and 2 b were obtained in benzene or toluene. At low temperatures, the same intermediates were observed in THF or toluene but the lower freezing point and position of the resonance signals of [D8]THF proved advantageous for the characterization of intermediates at low temperatures.
- 26The unusual shift for this aromatic proton could perhaps be explained by an agostic interaction with the palladium center or a through-space interaction with the O2 moiety.
- 27
- 27aJ. T. York, A. Llobet, C. J. Cramer, W. B. Tolman, J. Am. Chem. Soc. 2007, 129, 7990;
- 27bD. J. E. Spencer, A. M. Reynolds, P. L. Holland, B. A. Jazdzewski, C. Duboc-Toia, L. L. Pape, S. Yokota, Y. Tachi, S. Itoh, W. B. Tolman, Inorg. Chem. 2002, 41, 6307.
- 28J. S. Valentine, Chem. Rev. 1973, 73, 235.
- 29
- 29aC. J. Cramer, W. B. Tolman, K. H. Theopold, A. L. Rheingold, Proc. Natl. Acad. Sci. USA 2003, 100, 3635;
- 29bC. Cramer, W. Tolman, Acc. Chem. Res. 2007, 40, 601.
- 30C. R. Landis, C. M. Morales, S. S. Stahl, J. Am. Chem. Soc. 2004, 126, 16302.
- 31B. V. Popp, J. E. Wendlandt, C. R. Landis, S. S. Stahl, Angew. Chem. 2007, 119, 607;
10.1002/ange.200603667 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 601.
- 32A. D. Becke, J. Chem. Phys. 1993, 98, 5648.
- 33The open- and closed-shell singlets were higher in energy by 5 and 15 kcal mol−1, respectively.
- 34Relatively short PdH distances (2.3–2.6 Å) were observed in both intermediates A and B.
- 35H. Fan, B. C. Fullmer, M. Pink, K. G. Caulton, Angew. Chem. 2008, 120, 9252; Angew. Chem. Int. Ed. 2008, 47, 9112.
- 36P. E. Garrou, Chem. Rev. 1981, 81, 229.
- 37W. J. Tenn, K. J. H. Young, G. Bhalla, J. Oxgaard, W. A. Goddard, R. A. Periana, J. Am. Chem. Soc. 2005, 127, 14172.
- 38S. Cenini, F. Porta, M. Pizzotti, J. Organomet. Chem. 1985, 296, 291.
- 39For example see:
- 39aJ. L. Look, D. D. Wick, J. M. Mayer, K. I. Goldberg, Inorg. Chem. 2009, 48, 1356;
- 39bM. C. Denney, N. A. Smythe, K. L. Cetto, R. A. Kemp, K. I. Goldberg, J. Am. Chem. Soc. 2006, 128, 2508;
- 39cV. V. Rostovtsev, L. M. Henling, J. A. Labinger, J. E. Bercaw, Inorg. Chem. 2002, 41, 3608.
- 40J. Ho, A. Klamt, M. L. Coote, J. Phys. Chem.A 2010, 114, 13442.