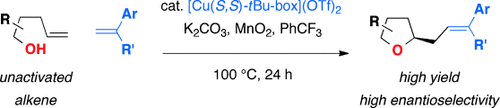Enantioselective Copper-Catalyzed Carboetherification of Unactivated Alkenes†
The National Institutes of Health (NIGMS RO1 078383), the donors of the American Chemical Society Petroleum Research Fund (51672-DNI6), and the Center for Computational Research at SUNY Buffalo are acknowledged for support of this research. We thank William W. Brennessel and the Crystallographic Facility at the University of Rochester for obtaining the X-ray structure of 2 b (CCDC 985356).
Graphical Abstract
A general scheme: A highly enantioselective copper-catalyzed carboetherification of 4-pentenols has been developed. Both intramolecular (formal CH functionalization) and intermolecular (net alkyl Heck-type coupling; see scheme) CC bond formation can occur, thus forming a range of chiral functionalized tetrahydrofurans. DFT transition-state calculations provide a rationale for the observed asymmetric induction.
Abstract
Chiral saturated oxygen heterocycles are important components of bioactive compounds. Cyclization of alcohols onto pendant alkenes is a direct route to their synthesis, but few catalytic enantioselective methods enabling cyclization onto unactivated alkenes exist. Herein reported is a highly efficient copper-catalyzed cyclization of γ-unsaturated pentenols which terminates in CC bond formation, a net alkene carboetherification. Both intra- and intermolecular CC bond formations are demonstrated, thus yielding functionalized chiral tetrahydrofurans as well as fused-ring and bridged-ring oxabicyclic products. Transition-state calculations support a cis-oxycupration stereochemistry-determining step.