Skeleton Decoration of NHCs by Amino Groups and its Sequential Booster Effect on the Palladium-Catalyzed Buchwald–Hartwig Amination†
Yin Zhang
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, BP44099, 31077 Toulouse Cedex 4 (France) http://www.lcc-toulouse.fr/lcc/spip.php?article24
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
Search for more papers by this authorCorresponding Author
Dr. Vincent César
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, BP44099, 31077 Toulouse Cedex 4 (France) http://www.lcc-toulouse.fr/lcc/spip.php?article24
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, BP44099, 31077 Toulouse Cedex 4 (France) http://www.lcc-toulouse.fr/lcc/spip.php?article24Search for more papers by this authorGolo Storch
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, BP44099, 31077 Toulouse Cedex 4 (France) http://www.lcc-toulouse.fr/lcc/spip.php?article24
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
Search for more papers by this authorDr. Noël Lugan
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, BP44099, 31077 Toulouse Cedex 4 (France) http://www.lcc-toulouse.fr/lcc/spip.php?article24
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
Search for more papers by this authorCorresponding Author
Dr. Guy Lavigne
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, BP44099, 31077 Toulouse Cedex 4 (France) http://www.lcc-toulouse.fr/lcc/spip.php?article24
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, BP44099, 31077 Toulouse Cedex 4 (France) http://www.lcc-toulouse.fr/lcc/spip.php?article24Search for more papers by this authorYin Zhang
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, BP44099, 31077 Toulouse Cedex 4 (France) http://www.lcc-toulouse.fr/lcc/spip.php?article24
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
Search for more papers by this authorCorresponding Author
Dr. Vincent César
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, BP44099, 31077 Toulouse Cedex 4 (France) http://www.lcc-toulouse.fr/lcc/spip.php?article24
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, BP44099, 31077 Toulouse Cedex 4 (France) http://www.lcc-toulouse.fr/lcc/spip.php?article24Search for more papers by this authorGolo Storch
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, BP44099, 31077 Toulouse Cedex 4 (France) http://www.lcc-toulouse.fr/lcc/spip.php?article24
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
Search for more papers by this authorDr. Noël Lugan
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, BP44099, 31077 Toulouse Cedex 4 (France) http://www.lcc-toulouse.fr/lcc/spip.php?article24
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
Search for more papers by this authorCorresponding Author
Dr. Guy Lavigne
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, BP44099, 31077 Toulouse Cedex 4 (France) http://www.lcc-toulouse.fr/lcc/spip.php?article24
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, BP44099, 31077 Toulouse Cedex 4 (France) http://www.lcc-toulouse.fr/lcc/spip.php?article24Search for more papers by this authorWe thank the CNRS for financial support and the Chinese Scholarship Council (CSC) for a PhD grant to Y.Z. NHCs=N-Heterocyclic Carbenes.
Graphical Abstract
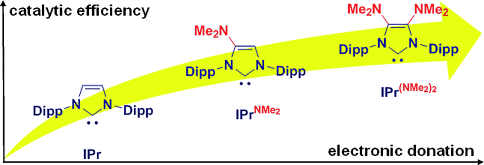
Boosting the PEPPSI: A sequential enhancement of the activity of the PEPPSI NHC-based palladium pre-catalyst in the Buchwald–Hartwig amination was obtained by a rational modification of its standard N-heterocyclic carbene (IMes or IPr), consisting of a “simple” incorporation of one, and then two, dimethylamino groups as substituents. These results highlight the valuable benefits of NHC-skeleton decoration in C–N cross-coupling.
Abstract
A challenging synthetic modification of PEPPSI-type palladium pre-catalysts consisting of a stepwise incorporation of one and two amino groups onto the NHC skeleton was seen to exert a sequential enhancement of the electronic donor properties. This appears to be positively correlated with the catalytic performances of the corresponding complexes in the Buchwald–Hartwig amination. This is illustrated, for example, by the quantitative amination of 4-chloroanisole by morpholine within 2 h at 25 °C with a 2 mol % catalyst/substrate ratio or by a significant reduction of catalytic loading (down to 0.005 mol %) for the coupling of aryl chlorides with anilines (max TON: 19 600).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201402301_sm_miscellaneous_information.pdf4.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For a recent book on NHCs, see:
- 1aRSC catalysis series N-Heterocyclic Carbenes (Ed.: ), RSC Publishing, Cambridge, 2011; For selected Reviews on NHCs as ligands in TM catalysis, see:
- 1bD. Bézier, J.-B. Sortais, C. Darcel, Adv. Synth. Catal. 2013, 355, 19;
- 1cC. Valente, S. Çalimsiz, K. H. Hoi, D. Mallik, M. Sayah, M. G. Organ, Angew. Chem. 2012, 124, 3370;
10.1002/ange.201106131 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 3314;
- 1dG. C. Vougioukalakis, R. H. Grubbs, Chem. Rev. 2010, 110, 1746;
- 1eS. Díez-González, N. Marion, S. P. Nolan, Chem. Rev. 2009, 109, 3612.
- 2Recent Reviews on the stereoelectronic properties of NHCs:
- 2aD. J. Nelson, S. P. Nolan, Chem. Soc. Rev. 2013, 42, 6723;
- 2bT. Dröge, F. Glorius, Angew. Chem. 2010, 122, 7094;
10.1002/ange.201001865 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 6940.
- 3
- 3aM. G. Organ, S. Çalimsiz, M. Sayah, K. H. Hoi, A. J. Lough, Angew. Chem. 2009, 121, 2419; Angew. Chem. Int. Ed. 2009, 48, 2383;
- 3bsee Ref. [1c];
- 3cA. Chartoire, X. Frogneux, S. P. Nolan, Adv. Synth. Catal. 2012, 354, 1897;
- 3dS. Meiries, A. Chartoire, A. M. Z. Slawin, S. P. Nolan, Organometallics 2012, 31, 3402;
- 3eA. Chartoire, X. Frogneux, A. Boreux, A. M. Z. Slawin, S. P. Nolan, Organometallics 2012, 31, 6947;
- 3fA. Chartoire, M. Lesieur, L. Falivene, A. M. Z. Slawin, L. Cavallo, C. S. J. Cazin, S. P. Nolan, Chem. Eur. J. 2012, 18, 4517;
- 3gS. Meiries, G. Le Duc, A. Chartoire, A. Collado, K. Speck, K. S. A. Arachchige, A. M. Z. Slawin, S. P. Nolan, Chem. Eur. J. 2013, 19, 17358.
- 4A. Albright, R. E. Gawley, J. Am. Chem. Soc. 2011, 133, 19680.
- 5Review on the synthetic routes to NHC precursors: L. Benhamou, E. Chardon, G. Lavigne, S. Bellemin-Laponnaz, V. César, Chem. Rev. 2011, 111, 2705.
- 6
- 6aA. G. Tennyson, V. M. Lynch, C. W. Bielawski, J. Am. Chem. Soc. 2010, 132, 9420;
- 6bM. D. Sanderson, J. W. Kamplain, C. W. Bielawski, J. Am. Chem. Soc. 2006, 128, 16514.
- 7
- 7aT. Tu, Z. Sun, W. Fang, M. Xu, Y. Zhou, Org. Lett. 2012, 14, 4250;
- 7bT. Tu, W. Fang, J. Jiang, Chem. Commun. 2011, 47, 12358;
- 7ca bis-NHC system derived from this structure has been recently studied in catalysis, see: G. Guisado-Barrios, J. Hiller, E. Peris, Chem. Eur. J. 2013, 19, 10405.
- 8
- 8aK. H. Hoi, J. A. Coggan, M. G. Organ, Chem. Eur. J. 2013, 19, 843;
- 8bM. Pompeo, R. D. J. Froese, N. Hadei, M. G. Organ, Angew. Chem. 2012, 124, 11516;
10.1002/ange.201205747 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 11354;
- 8cC. A. Urbina-Blanco, X. Bantreil, H. Clavier, A. M. Z. Slawin, S. P. Nolan, Beilstein J. Org. Chem. 2010, 6, 1120.
- 9
- 9aE. L. Kolychev, S. Kronig, K. Brandhorst, M. Freytag, P. G. Jones, M. Tamm, J. Am. Chem. Soc. 2013, 135, 12448;
- 9bS. Kronig, E. Theuergarten, C. G. Daniliuc, P. G. Jones, M. Tamm, Angew. Chem. 2012, 124, 3294;
10.1002/ange.201108813 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 3240.
- 10Selected references:
- 10aV. César, S. Labat, K. Miqueu, J.-M. Sotiropoulos, R. Brousses, N. Lugan, G. Lavigne, Chem. Eur. J. 2013, 19, 17113;
- 10bV. César, J. C. Tourneux, N. Vujkovic, R. Brousses, N. Lugan, G. Lavigne, Chem. Commun. 2012, 48, 2349;
- 10cN. Vujkovic, V. César, N. Lugan, G. Lavigne, Chem. Eur. J. 2011, 17, 13151;
- 10dV. César, N. Lugan, G. Lavigne, Chem. Eur. J. 2010, 16, 11432;
- 10eL. Benhamou, V. César, H. Gornitzka, N. Lugan, G. Lavigne, Chem. Commun. 2009, 4720;
- 10fV. César, N. Lugan, G. Lavigne, J. Am. Chem. Soc. 2008, 130, 11286.
- 114,5-bis(dimethylamino)imidazol-2-ylidenes have been reported but only with non-hindered aryl groups as nitrogen arms. See: S. M. Huber, F. W. Heinemann, P. Audebert, R. Weiss, Chem. Eur. J. 2011, 17, 13078.
- 12CCDC 984192, 984193, 984194, 984195, 984196, 984197, 984198 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 13The molecular structure of 15 is depicted in the Supporting Information.
- 14The TEP value was calculated using the linear correlations developed by Nolan and Plenio, see:
- 14aR. A. Kelly III, H. Clavier, S. Giudice, N. M. Scott, E. D. Stevens, J. Bordner, I. Samardjiev, C. D. Hoff, L. Cavallo, S. P. Nolan, Organometallics 2008, 27, 202;
- 14bS. Wolf, H. Plenio, J. Organomet. Chem. 2009, 694, 1487. For a pioneering work, see:
- 14cA. R. Chianese, X. Li, M. C. Jansen, J. W. Faller, R. H. Crabtree, Organometallics 2003, 22, 1663.
- 15PEPPSI:
- 15asee Ref. [8a];
- 15bsee Ref. [8b];
- 15csee Ref. [1c], and references therein.
- 16For a Review, see:
- 16aH. Clavier, S. P. Nolan, Chem. Commun. 2010, 46, 841; See also:
- 16bA. Poater, B. Cosenza, A. Correa, S. Giudice, F. Ragone, V. Scarano, L. Cavallo, Eur. J. Inorg. Chem. 2009, 1759;
- 16cL. Cavallo, A. Correa, C. Costabile, H. Jacobsen, J. Organomet. Chem. 2005, 690, 5407.
- 17Vbur were calculated using the SambVca web application (https://www.molnac.unisa.it/OMtools/sambvca.php) with the following parameters: sphere radius: 3.5 Å, distance from the center of the sphere: 2.00 Å, mesh spacing: 0.05 Å, hydrogen atoms not included.
- 18
- 18aJ. F. Hartwig in Modern Amination Methods, Wiley-VCH, Weinheim, 2000, p. 195;
10.1002/9783527613182.ch7 Google Scholar
- 18bJ. F. Hartwig in Handbook of Organopalladium Chemistry for Organic Synthesis, Wiley, New York, 2002, p. 1051;
10.1002/0471212466.ch42 Google Scholar
- 18cL. Jiang, S. L. Buchwald in Metal-Catalyzed Cross-Coupling Reactions, Wiley-VCH, Weinheim, 2004, p. 699;
- 18dD. S. Surry, S. L. Buchwald, Angew. Chem. 2008, 120, 6438;
10.1002/ange.200800497 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6338.
- 19
- 19aK. H. Hoi, S. Çalimsiz, R. D. J. Froese, A. C. Hopkinson, M. G. Organ, Chem. Eur. J. 2012, 18, 145;
- 19bG. Le Duc, S. Meiries, S. P. Nolan, Organometallics 2013, 32, 7547.
- 20
- 20aR. J. Lundgren, M. Stradiotto, Aldrichimica Acta 2012, 45, 59;
- 20bR. J. Lundgren, A. Sappong-Kumankumah, M. Stradiotto, Chem. Eur. J. 2010, 16, 1983;
- 20cB. P. Fors, S. L. Buchwald, J. Am. Chem. Soc. 2010, 132, 15914;
- 20dQ. Shen, T. Ogata, J. F. Hartwig, J. Am. Chem. Soc. 2008, 130, 6586.
- 21During the refereeing process of the present manuscript, the room-temperature coupling of deactivated anilines with aryl chlorides using a PEPPSI pre-catalyst and a mild base was reported: M. Pompeo, J. L. Farmer, R. D. J. Froese, M. G. Organ, Angew. Chem. 2014, 126, 3287;
10.1002/ange.201310457 Google ScholarAngew. Chem. Int. Ed. 2014, 53, 3223.