Highly Enantioselective Carbonyl–Ene Reactions of 2,3-Diketoesters: Efficient and Atom-Economical Process to Functionalized Chiral α-Hydroxy-β-Ketoesters†
Phong M. Truong
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742 (USA)
Search for more papers by this authorDr. Peter Y. Zavalij
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742 (USA)
Search for more papers by this authorCorresponding Author
Prof. Michael P. Doyle
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742 (USA)
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742 (USA)Search for more papers by this authorPhong M. Truong
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742 (USA)
Search for more papers by this authorDr. Peter Y. Zavalij
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742 (USA)
Search for more papers by this authorCorresponding Author
Prof. Michael P. Doyle
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742 (USA)
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742 (USA)Search for more papers by this authorSupport for this research from the National Science Foundation (CHE 1212446) is gratefully acknowledged.
Graphical Abstract
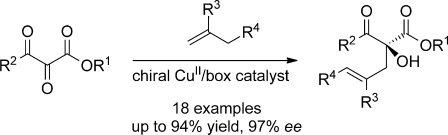
Outside the box: Carbonyl–ene reactions of 2,3-diketoesters, catalyzed by [Cu{(S,S)-tBu-box}](SbF6)2 [box=bis(oxazoline)], generate chiral α-functionalized α-hydroxy-β-ketoesters in up to 94 % yield and 97 % ee. The 2,3-diketoesters are conveniently accessed from the corresponding α-diazo-β-ketoester, and a catalyst loading as low as 1.0 mol % can be used.
Abstract
Carbonyl–ene reactions of 2,3-diketoesters catalyzed by [Cu{(S,S)-tBu-box}](SbF6)2 [box=bis(oxazoline)] generate chiral α-functionalized α-hydroxy-β-ketoesters in up to 94 % yield and 97 % ee. The 2,3-diketoesters are conveniently accessed from the corresponding α-diazo-β-ketoester, and a catalyst loading as low as 1.0 mol % can be achieved.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201402233_sm_miscellaneous_information.pdf5.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For a general review of the ene reaction, see:
- 1aK. Mikami, M. Shimizu, Chem. Rev. 1992, 92, 1021;
- 1bL. Carlos Dias, Curr. Org. Chem. 2000, 4, 305;
- 1cM. L. Clarke, M. B. France, Tetrahedron 2008, 64, 9003.
- 2K. Maruoka, Y. Hoshino, Y. H. Shirasaka, H. Yamamoto, Tetrahedron Lett. 1988, 29, 3967.
- 3For examples of asymmetric carbonyl–ene reaction of glyoxylate, see:
- 3aK. Mikami, M. Terada, T. Nakai, J. Am. Chem. Soc. 1989, 111, 1940;
- 3bK. Mikami, M. Terada, T. Nakai, J. Am. Chem. Soc. 1990, 112, 3949;
- 3cK. Mikami, S. Matsukawa, J. Am. Chem. Soc. 1993, 115, 7039;
- 3dD. A. Evans, C. S. Burgey, N. A. Paras, T. Vojkovsky, S. W. Tregay, J. Am. Chem. Soc. 1998, 120, 5824;
- 3eJ. Hao, M. Hatano, K. Mikami, Org. Lett. 2000, 2, 4059;
- 3fS. Pandiaraju, G. Chen, A. Lough, A. K. Yudin, J. Am. Chem. Soc. 2001, 123, 3850;
- 3gJ. H. Koh, A. O. Larsen, M. R. Gagne, Org. Lett. 2001, 3, 1233;
- 3hK. Ding, Y. Yuan, X. Zhang, Angew. Chem. 2003, 115, 5636; Angew. Chem. Int. Ed. 2003, 42, 5478;
- 3iG. E. Hutson, A. H. Dave, V. H. Rawal, Org. Lett. 2007, 9, 3869;
- 3jJ. F. Zhao, H. Y. Tsui, P. J. Wu, J. Lu, T. P. Loh, J. Am. Chem. Soc. 2008, 130, 16492.
- 4For examples of asymmetric carbonyl–ene reactions of trifluoromethylpyruvate derivatives, see:
- 4aK. Aikawa, S. Kainuma, M. Hatano, K. Mikami, Tetrahedron Lett. 2004, 45, 183;
- 4bS. Doherty, J. G. Knight, C. H. Smyth, R. W. Harrington, W. Clegg, J. Org. Chem. 2006, 71, 9751;
- 4cH. K. Luo, Y. L. Woo, H. Schumann, C. Jacob, M. Meurs, H. Y. Yang, Y. T. Tan, Adv. Synth. Catal. 2010, 352, 1356;
- 4dJ. F. Zhao, B. H. Tan, M. K. Zhu, T. B. W. Tjan, T. P. Loh, Adv. Synth. Catal. 2010, 352, 2085;
- 4eT. Wang, X. Q. Hao, J. J. Huang, J. L. Niu, J. F. Gong, M. P. Song, J. Org. Chem. 2013, 78, 8712.
- 5For examples of asymmetric carbonyl–ene reaction of pyruvate derivatives, see:
- 5aD. A. Evans, S. W. Tregay, C. S. Burgey, N. A. Paras, T. Vojkovsky, J. Am. Chem. Soc. 2000, 122, 7936;
- 5bK. Mikami, Y. Kawakami, K. Akiyama, K. Aikawa, J. Am. Chem. Soc. 2007, 129, 12950.
- 6For asymmetric glyoxal–ene reactions, see:
- 6aS. Kezuka, T. Ikeno, T. Yamada, Org. Lett. 2001, 3, 1937;
- 6bH. K. Luo, L. B. Khim, H. Schumann, C. Lim, T. X. Jie, H. Y. Yang, Adv. Synth. Catal. 2007, 349, 1781;
- 6cK. Zheng, J. Shi, X. Liu, X. Feng, J. Am. Chem. Soc. 2008, 130, 15770.
- 7For other examples of asymmetric carbonyl–ene reactions, see:
- 7aE. M. Carreira, W. Lee, R. A. Singer, J. Am. Chem. Soc. 1995, 117, 3649;
- 7bK. Mikami, T. Yajima, T. Takasaki, S. Matsukawa, M. Terada, T. Uchimaru, M. Maruta, Tetrahedron 1996, 52, 85;
- 7cR. T. Ruck, E. N. Jacobsen, J. Am. Chem. Soc. 2002, 124, 2882;
- 7dR. T. Ruck, E. N. Jacobsen, Angew. Chem. 2003, 115, 4919;
10.1002/ange.200351591 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4771;
- 7eD. A. Evans, J. Wu, J. Am. Chem. Soc. 2005, 127, 8006;
- 7fM. L. Grachan, M. T. Tudge, E. N. Jacobsen, Angew. Chem. 2008, 120, 1491;
10.1002/ange.200704439 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 1469.
- 8
- 8aM. Rueping, T. Theissmann, A. Kuenkel, R. M. Koenigs, Angew. Chem. 2008, 120, 6903;
10.1002/ange.200802139 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6798;
- 8bM. Terada, K. Soga, N. Momiyama, Angew. Chem. 2008, 120, 4190; Angew. Chem. Int. Ed. 2008, 47, 4122;
- 8cM. Rueping, T. Bootwicha, S. Kambutong, E. Sugiono, Chem. Asian J. 2012, 7, 1195.
- 9P. Truong, X. Xu, M. P. Doyle, Tetrahedron Lett. 2011, 52, 2093;
- 9bP. Truong, C. S. Shanahan, M. P. Doyle, Org. Lett. 2012, 14, 3608.
- 10
- 10aM. Nakayama, Y. Fukuoka, H. Nozaki, A. Matsuo, S. Hayashi, Chem. Lett. 1980, 1243;
- 10bJ. Zhu, A. J. H. Klunder, B. Zwanenburg, Tetrahedron Lett. 1994, 35, 2787;
- 10cK. D. Wellington, R. C. Cambie, P. S. Rutledge, P. R. Bergquist, J. Nat. Prod. 2000, 63, 79;
- 10dJ. Christoffers, A. Baro, T. Werner, Adv. Synth. Catal. 2004, 346, 143;
- 10eK. Tamaki, J. B. Shotwell, R. D. White, I. Drutu, D. T. Petsch, T. V. Nheu, H. He, Y. Hirokawa, H. Maruta, J. L. Wood, Org. Lett. 2001, 3, 1689;
- 10fJ. Li, G. Chen, Z. Wang, R. Zhang, X. Zhang, K. Ding, Chem. Sci. 2011, 2, 1141;
- 10gC. A. Rose, S. Gundala, C.-L. Fagan, J. F. Franz, S. J. Connon, K. Zietler, Chem. Sci. 2012, 3, 735;
- 10hM. Baidya, K. A. Griffin, H. Yamamoto, J. Am. Chem. Soc. 2012, 134, 18566;
- 10iJ. H. Chen, S. R. Levine, J. F. Buergler, T. C. McMahon, M. R. Medeiros, J. L. Wood, Org. Lett. 2012, 14, 4531;
- 10jR. Nagase, Y. Oguni, S. Ureshino, H. Mura, T. Misaki, Y. Tanabe, Chem. Commun. 2013, 49, 7001.
- 11
- 11aH. H. Wasserman, J. Parr, Acc. Chem. Res. 2004, 37, 687;
- 11bM. B. Rubin, R. Gleiter, Chem. Rev. 2000, 100, 1121.
- 12
- 12aB. M. Trost, S. Malhotra, P. Koschker, P. Ellerbrock, J. Am. Chem. Soc. 2012, 134, 2075;
- 12bD. L. J. Clive, Minaruzzaman, Org. Lett. 2007, 9, 5315;
- 12cM. A. Andersson, R. Epple, V. V. Fokin, K. B. Sharpless, Angew. Chem. 2002, 114, 490;
10.1002/1521-3757(20020201)114:3<490::AID-ANGE490>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 2002, 41, 472.10.1002/1521-3773(20020201)41:3<472::AID-ANIE472>3.0.CO;2-7 CAS PubMed Web of Science® Google Scholar
- 13J. S. Johnson, D. A. Evans, Acc. Chem. Res. 2000, 33, 325.