Ruthenium-Catalyzed trans-Selective Hydrostannation of Alkynes†
M. Sc. Stephan M. Rummelt
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)
Search for more papers by this authorCorresponding Author
Prof. Alois Fürstner
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)Search for more papers by this authorM. Sc. Stephan M. Rummelt
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)
Search for more papers by this authorCorresponding Author
Prof. Alois Fürstner
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany)Search for more papers by this authorGenerous financial support from the MPG and the Fonds der Chemischen Industrie is gratefully acknowledged. We thank Dr. B. Sundararaju for preliminary studies and Dr. C. Farès for NMR support.
Graphical Abstract
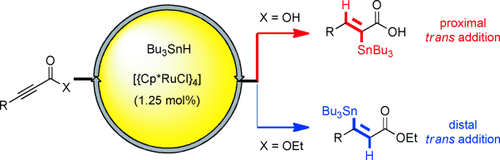
Unorthodox: Ruthenium catalysts allow stannanes to be added in a trans fashion across the triple bonds of terminal, internal, silylated, and chlorinated alkynes. This pattern violates the basic mechanism of transition-metal catalysis which otherwise secures high cis selectivity in hydrometalations. Cooperative effects between the ruthenium species and protic functionality render reactions of unsymmetrical substrates regioselective. Cp*=η5-C5Me5.
Abstract
In contrast to all other transition-metal-catalyzed hydrostannation reactions documented in the literature, the addition of Bu3SnH across various types of alkynes proceeds with excellent trans selectivity, provided the reaction is catalyzed by [Cp*Ru]-based complexes. This method is distinguished by a broad substrate scope and a remarkable compatibility with functional groups, including various substituents that would neither survive under the conditions of established Lewis acid mediated trans hydrostannations nor withstand free-radical reactions. In case of unsymmetrical alkynes, a cooperative effect between the proper catalyst and protic functionality in the substrate allows outstanding levels of regioselectivity to be secured as well.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201311080_sm_miscellaneous_information.pdf4.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1K. Radkowski, B. Sundararaju, A. Fürstner, Angew. Chem. 2013, 125, 373–378;
10.1002/ange.201205946 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 355–360.
- 2B. Sundararaju, A. Fürstner, Angew. Chem. 2013, 125, 14300–14304;
10.1002/ange.201307584 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 14050–14054.
- 3
- 3aB. M. Trost, Z. T. Ball, T. Jöge, J. Am. Chem. Soc. 2002, 124, 7922–7923;
- 3bB. M. Trost, Z. T. Ball, J. Am. Chem. Soc. 2005, 127, 17644–17655;
- 3cB. M. Trost, M. R. Machacek, Z. T. Ball, Org. Lett. 2003, 5, 1895–1898;
- 3dB. M. Trost, Z. T. Ball, J. Am. Chem. Soc. 2003, 125, 30–31.
- 4
- 4aA. Fürstner, K. Radkowski, Chem. Commun. 2002, 2182–2183;
- 4bF. Lacombe, K. Radkowski, G. Seidel, A. Fürstner, Tetrahedron 2004, 60, 7315–7324.
- 5For a related trans hydrogermylation, see: T. Matsuda, S. Kadowaki, Y. Yamaguchi, M. Murakami, Org. Lett. 2010, 12, 1056–1058.
- 6G. J. Kubas, Metal Dihydrogen and σ-Bond Complexes, Kluwer/Plenum, Dordrecht, 2001.
10.1007/b113929 Google Scholar
- 7aG. J. Kubas, Catal. Lett. 2005, 104, 79–101;
- 7bS. Lachaize, S. Szabo-Etienne, Eur. J. Inorg. Chem. 2006, 2115–2127;
- 7cR. H. Crabtree, Angew. Chem. 1993, 105, 828–845; Angew. Chem. Int. Ed. Engl. 1993, 32, 789–805.
- 8For another recent study into a σ-silane complex relevant to catalysis, see: D. V. Gutsulyak, S. F. Vyboishchikov, G. I. Nikonov, J. Am. Chem. Soc. 2010, 132, 5950–5951.
- 9L. W. Chung, Y.-D. Wu, B. M. Trost, Z. T. Ball, J. Am. Chem. Soc. 2003, 125, 11578–11582.
- 10For the hydrosilylation, computations suggest that hydride delivery precedes silyl delivery (see Ref. [9]); we assume that the same phasing applies to the trans hydroboration, although a reverse order of events cannot be excluded at this point. Whether differences in the regioselective outcomes of trans hydrostannations, hydrosilylations, and hydroborations are caused by changes in the timing of these elementary steps is speculative at this point.
- 11D. S. Frohnapfel, J. L. Templeton, Coord. Chem. Rev. 2000, 206–207, 199–235.
- 12A. Fürstner, M. Bonnekessel, J. T. Blank, K. Radkowski, G. Seidel, F. Lacombe, B. Gabor, R. Mynott, Chem. Eur. J. 2007, 13, 8762–8783.
- 13For a striking case in which replacement of Cp* by Cp affected the stereo- as well as the regioselectivity of alkyne hydrosilylations, see: S. Ding, L.-J. Song, L. W. Chung, X. Zhang, J. Sun, Y.-D. Wu, J. Am. Chem. Soc. 2013, 135, 13835–13842.
- 14Reviews:
- 14aN. D. Smith, J. Mancuso, M. Lautens, Chem. Rev. 2000, 100, 3257–3282;
- 14bN. Asao, Y. Yamamoto, Bull. Chem. Soc. Jpn. 2000, 73, 1071–1087;
- 14cB. M. Trost, Z. T. Ball, Synthesis 2005, 853–887.
- 15
- 15aU. Schubert, E. Kunz, B. Harkers, J. Willnecker, J. Meyer, J. Am. Chem. Soc. 1989, 111, 2572–2574;
- 15bH. Piana, U. Kirchgäßner, U. Schubert, Chem. Ber. 1991, 124, 743–751;
- 15cL. Carlton, Inorg. Chem. 2000, 39, 4510–4519;
- 15dL. Carlton, R. Weber, D. C. Levendis, Inorg. Chem. 1998, 37, 1264–1271;
- 15eA. Khaleel, K. J. Klabunde, Inorg. Chem. 1996, 35, 3223–3227;
- 15fL. Carlton, M. A. Fernandes, E. Sitabule, Proc. Natl. Acad. Sci. USA 2007, 104, 6969–6973.
- 16
- 16aJ. Heppekausen, R. Stade, R. Goddard, A. Fürstner, J. Am. Chem. Soc. 2010, 132, 11045–11057;
- 16bJ. Heppekausen, R. Stade, A. Kondoh, G. Seidel, R. Goddard, A. Fürstner, Chem. Eur. J. 2012, 18, 10281–10299.
- 17A. Fürstner, Angew. Chem. 2013, 125, 2860–2887;
10.1002/ange.201204513 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 2794–2819.
- 18The trans-addition mode is evident from the spectral data; see the Supporting Information. Particularly diagnostic is the JSn,H coupling constant, which is about twice as large if tin and hydrogen are trans to each other than if they are in a cis arrangement: B. Wrackmeyer, Annu. Rep. NMR Spectrosc. 1986, 24, 73–186.
- 19Commercial Bu3SnH is stabilized with 0.05 % of 3,5-di-tert-butyl-4-hydroxytoluene, which was not removed in any of the reactions described herein.
- 20Distannane formation plagues many transition-metal-catalyzed hydrostannations. For a countermeasure, see: M. F. Semmelhack, R. J. Hooley, Tetrahedron Lett. 2003, 44, 5737–5739.
- 21Arenes readily coordinate in a η6-fashion to [LRu]+ (L=Cp, Cp*):
- 21aT. P. Gill, K. R. Mann, Organometallics 1982, 1, 485–488;
- 21bA. Schmid, H. Piotrowski, T. Lindel, Eur. J. Inorg. Chem. 2003, 2255–2263.
- 22
- 22aN. Asao, J.-X. Liu, T. Sudoh, Y. Yamamoto, J. Org. Chem. 1996, 61, 4568–4571;
- 22bsee also: M. S. Oderinde, M. G. Organ, Angew. Chem. 2012, 124, 9972–9975;
10.1002/ange.201204060 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 9834–9837.
- 23The related trans-hydrosilylation of propargylic alcohols catalyzed by 3 favors β-silylation: B. M. Trost, Z. T. Ball, T. Jöge, Angew. Chem. 2003, 115, 3537–3540;
10.1002/ange.200351587 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 3415–3418.
- 24P. J. Fagan, W. S. Mahoney, J. C. Calabrese, I. D. Williams, Organometallics 1990, 9, 1843–1852.
- 25For representative cases, see:
- 25aH. X. Zhang, F. Guibé, G. Balavoine, J. Org. Chem. 1990, 55, 1857–1867;
- 25bJ.-F. Betzer, F. Delaloge, B. Muller, A. Pancrazi, J. Prunet, J. Org. Chem. 1997, 62, 7768–7780;
- 25cF. Liron, P. Le Garrec, M. Alami, Synlett 1999, 246–248;
- 25dJ. A. Marshall, M. P. Bourbeau, Tetrahedron Lett. 2003, 44, 1087–1089;
- 25eN. Greeves, J. S. Torode, Synlett 1994, 537–538.
- 26Specific examples are contained in the Supporting Information.
- 27
- 27aIt is presently unclear whether the relevant species derived from 7 and Bu3SnH is monomeric or multinuclear. In any case, even bimetallic ruthenium species can form catalytically active σ-complexes with H2: A. M. Joshi, B. R. James, J. Chem. Soc. Chem. Commun. 1989, 1785–1786;
- 27bA. M. Joshi, K. S. MacFarlane, B. R. James, J. Organomet. Chem. 1995, 488, 161–167;
- 27cfor a review on secondary interactions in σ-complexes, see Ref. [7b];
- 27dfor a noteworthy interligand contact within a σ-complex, see: A. L. Osipov, S. F. Vyboishchikov, K. Y. Dorogov, L. G. Kuzmina, J. A. K. Howard, D. A. Lemenovskii, G. I. Nikonov, Chem. Commun. 2005, 3349–3351.
- 28For an interesting example of a neutral 16-electron cyclopentadienyl half sandwich complex which features an internal hydrogen bond between a coordinated propargyl alcohol and the chloride ligand on ruthenium, see: B. Dutta, B. F. E. Curchod, P. Campomanes, E. Solari, R. Scopelliti, U. Rothlisberger, K. Severin, Chem. Eur. J. 2010, 16, 8400–8409.
- 29In radical additions of R3SnH, the cis/trans ratio is strongly influenced by secondary processes caused by the tin radicals. Propargylic alcohols usually favor α-stannylation via O-complexed tin radicals, although a recent investigation speaks for a more involved mechanism. For representative studies, see:
- 29aA. J. Leusink, H. A. Budding, J. Organomet. Chem. 1968, 11, 533–539;
- 29bC. Nativi, M. Taddei, J. Org. Chem. 1988, 53, 820–826;
- 29cP. Dimopoulos, J. George, D. A. Tocher, S. Manaviazar, K. J. Hale, Org. Lett. 2005, 7, 5377–5380;
- 29dM. S. Oderinde, R. D. J. Froese, M. G. Organ, Angew. Chem. 2013, 125, 11544–11548;
10.1002/ange.201303736 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 11334–11338.
- 30For representative studies, see Ref. [25a] and the following:
- 30aJ. C. Cochran, B. S. Bronk, K. M. Terrence, H. K. Phillips, Tetrahedron Lett. 1990, 31, 6621–6624;
- 30bU. Kazmaier, M. Pohlmann, D. Schauss, Eur. J. Org. Chem. 2000, 2761–2766.
- 31For terminal alkynes, a slightly modified procedure was used, in which a solution of the substrate and Bu3SnH in CH2Cl2 was slowly added to a solution of the catalyst in the same solvent; see the Supporting Information.
- 32With other transition-metal catalysts, the regioselectivity can vary from modest to excellent. For leading references, see Ref. [25a, 30b] and the following:
- 32aK. Kikukawa, H. Umekawa, F. Wada, T. Matsuda, Chem. Lett. 1988, 881–884;
- 32bY. Ichinose, H. Oda, K. Oshima, K. Utimoto, Bull. Chem. Soc. Jpn. 1987, 60, 3468–3470.
- 33
- 33aM. Pereyre, J.-P. Quintard, A. Rahm, Tin in Organic Synthesis Butterworths, Stoneham, Mass., 1986;
- 33b Main Group Metals in Organic Synthesis, Vol. 1,2 (Eds.: ), Wiley-VCH, Weinheim, 2004;
- 33cV. Farina, V. Krishnamurthy, W. J. Scott, Org. React. 1997, 50, 1–652.
- 34For an instructive case from our laboratory, in which a Suzuki coupling, though optimized, was ultimately replaced by a Stille reaction, see:
- 34aJ. Gagnepain, E. Moulin, A. Fürstner, Chem. Eur. J. 2011, 17, 6964–6972;
- 34bA. Fürstner, J.-A. Funel, M. Tremblay, L. C. Bouchez, C. Nevado, M. Waser, J. Ackerstaff, Chem. Commun. 2008, 2873–2875.
- 35For recent total syntheses based upon alkyne trans-addition chemistry reported by our group, see:
- 35aK. Micoine, A. Fürstner, J. Am. Chem. Soc. 2010, 132, 14064–14066;
- 35bK. Lehr, R. Mariz, L. Leseurre, B. Gabor, A. Fürstner, Angew. Chem. 2011, 123, 11575–11579;
10.1002/ange.201106117 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 11373–11377;
- 35cK. Micoine, P. Persich, J. Llaveria, M.-H. Lam, A. Maderna, F. Loganzo, A. Fürstner, Chem. Eur. J. 2013, 19, 7370–7383.