Unexpected Formation of a [4]Radialene and Dendralenes by Addition of Tetracyanoethylene to a Tetraaryl[5]cumulene†
Johanna A. Januszewski
Department für Chemie und Pharmazie & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Henkestrasse 42, 91054 Erlangen (Germany) http://www.chemie.uni-erlangen.de/tykwinski
Search for more papers by this authorDr. Frank Hampel
Department für Chemie und Pharmazie & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Henkestrasse 42, 91054 Erlangen (Germany) http://www.chemie.uni-erlangen.de/tykwinski
Search for more papers by this authorDr. Christian Neiss
Lehrstuhl für Theoretische Chemie & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Egerlandstrasse 3, 91058 Erlangen (Germany)
Search for more papers by this authorProf. Dr. Andreas Görling
Lehrstuhl für Theoretische Chemie & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Egerlandstrasse 3, 91058 Erlangen (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Rik R. Tykwinski
Department für Chemie und Pharmazie & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Henkestrasse 42, 91054 Erlangen (Germany) http://www.chemie.uni-erlangen.de/tykwinski
Department für Chemie und Pharmazie & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Henkestrasse 42, 91054 Erlangen (Germany) http://www.chemie.uni-erlangen.de/tykwinskiSearch for more papers by this authorJohanna A. Januszewski
Department für Chemie und Pharmazie & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Henkestrasse 42, 91054 Erlangen (Germany) http://www.chemie.uni-erlangen.de/tykwinski
Search for more papers by this authorDr. Frank Hampel
Department für Chemie und Pharmazie & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Henkestrasse 42, 91054 Erlangen (Germany) http://www.chemie.uni-erlangen.de/tykwinski
Search for more papers by this authorDr. Christian Neiss
Lehrstuhl für Theoretische Chemie & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Egerlandstrasse 3, 91058 Erlangen (Germany)
Search for more papers by this authorProf. Dr. Andreas Görling
Lehrstuhl für Theoretische Chemie & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Egerlandstrasse 3, 91058 Erlangen (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Rik R. Tykwinski
Department für Chemie und Pharmazie & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Henkestrasse 42, 91054 Erlangen (Germany) http://www.chemie.uni-erlangen.de/tykwinski
Department für Chemie und Pharmazie & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Henkestrasse 42, 91054 Erlangen (Germany) http://www.chemie.uni-erlangen.de/tykwinskiSearch for more papers by this authorFunding is gratefully acknowledged from the Friedrich-Alexander-Universität Erlangen-Nürnberg, the Deutsche Forschungsgemeinschaft (SFB 953, “Synthetic Carbon Allotropes”), and “Solar Technologies go Hybrid”—an initiative of the Bavarian State Ministry for Science, Research, and Art. We thank Dr. Alexandra Griffin (Agilent Technologies) for collecting the X-ray structure data of 10 a.
Graphical Abstract
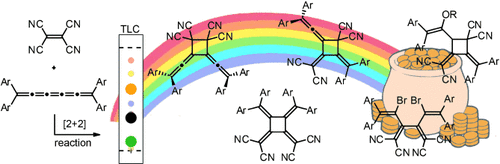
Unusual structures and interesting electronic properties characterize the cyclic products from the reaction of TCNE with tetraaryl[5]cumulene. Mechanistic investigations, aided by DFT calculations, outline a likely pathway to the observed products. The addition of MeOH, EtOH, and Br2 to various intermediates leads to the interesting dendralene compounds.
Abstract
The use of cumulenes in synthetic transformations offers the possibility to form structurally interesting and potentially useful conjugated molecules. The cycloaddition reaction of a tetraaryl[5]cumulene with the electron-deficient olefin tetracyanoethylene affords unusual products, including functionalized dendralenes and alkylidene cyclobutanes, as well as a symmetric [4]radialene that shows unique solvatochromism, with λmax values approaching the near-IR region. These carbon-rich products have been investigated spectroscopically and by X-ray crystallographic analysis (five structures). The cycloaddition reaction sequence has also been explored by mechanistic and theoretical studies. The obtained results clearly demonstrate the potential of [5]cumulenes to serve as precursors for unprecedented conjugated structures.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201309355_sm_miscellaneous_information.pdf1.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aH. Hopf, Classics in Hydrocarbon Chemistry, Wiley-VCH, Weinheim, 2000, Chap. 9;
- 1bH. Fischer in The Chemistry of Alkenes (Ed.: ), Wiley, New York, 1964, pp. 1025–1159.
- 2See, for example: I. E. Castelli, P. Salvestrini, N. Manini, Phys. Rev. B 2012, 85, 214110; B. Akdim, R. Pachter, ACS Nano 2011, 5, 1769–1774; C. H. Hendon, D. Tiana, A. T. Murray, D. R. Carbery, A. Walsh, Chem. Sci. 2013, 4, 4278–4284.
- 3P. Rivera-Fuentes, F. Diederich, Angew. Chem. 2012, 124, 2872–2882;
10.1002/ange.201108001 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 2818–2828; S. Yu, S. Ma, Angew. Chem. 2012, 124, 3128–3167; Angew. Chem. Int. Ed. 2012, 51, 3074–3112.
- 4L. Leroyer, V. Maraval, R. Chauvin, Chem. Rev. 2012, 112, 1310–1343.
- 5A. Auffrant, F. Diederich, C. Boudon, J.-P. Gisselbrecht, M. Gross, Helv. Chim. Acta 2004, 87, 3085–3105.
- 6J. A. Januszewski, D. Wendinger, C. D. Methfessel, F. Hampel, R. R. Tykwinski, Angew. Chem. 2013, 125, 1862–1867;
10.1002/ange.201208058 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 1817–1821.
- 7H. D. Hartzler, J. Am. Chem. Soc. 1971, 93, 4527–4531.
- 8Y. Kuwatani, G. Yamamoto, M. Oda, M. Iyoda, Bull. Chem. Soc. Jpn. 2005, 78, 2188–2208.
- 9N. Islam, T. Ooi, T. Iwasawa, M. Nishiuchia, Y. Kawamura, Chem. Commun. 2009, 574–576.
- 10A cyclotrimerization, at the β-bonds of a [5]cumulene, has also been reported, see T. Kawase, T. Minami, N. Nishigaki, S. Okano, H. Kurata, M. Oda, Angew. Chem. 2004, 117, 320–323;
10.1002/ange.200461608 Google ScholarAngew. Chem. Int. Ed. 2004, 44, 316–319.
- 11B. Bildstein, M. Schweiger, H. Angleitner, H. Kopacka, K. Wurst, K.-H. Ongania, M. Fontani, P. Zanello, Organometallics 1999, 18, 4286–4295.
- 12M. Kivala, F. Diederich, Acc. Chem. Res. 2009, 42, 235–248; S.-i. Kato, F. Diederich, Chem. Commun. 2010, 46, 1994–2006.
- 13M. Štefko, M. D. Tzirakis, B. Breiten, M.-O. Ebert, O. Dumele, W. B. Schweizer, J.-P. Gisselbrecht, C. Boudon, M. T. Beels, I. Biaggio, F. Diederich, Chem. Eur. J. 2013, 19, 12693–12704; Y.-L. Wu, F. Tancini, W. B. Schweizer, D. Paunescu, C. Boudon, J.-P. Gisselbrecht, P. D. Jarowski, E. Dalcanale, F. Diederich, Chem. Asian J. 2012, 7, 1185–1190; B. Breiten, Y.-L. Wu, P. D. Jarowski, J.-P. Gisselbrecht, C. Boudon, M. Griesser, C. Onitsch, G. Gescheidt, W. B. Schweizer, N. Langer, C. Lennartz, F. Diederich, Chem. Sci. 2011, 2, 88–93.
- 14For example, see Y. Li, M. Ashizawa, S. Uchida, T. Michinobu, Polym. Chem. 2012, 3, 1996–2005; T. Shoji, S. Ito, T. Okujima, N. Morita, Chem. Eur. J. 2013, 19, 5721–5730.
- 15M. Gholami, R. R. Tykwinski, Chem. Rev. 2006, 106, 4997–5027.
- 16For leading references on dendralenes, see
- 16aA. D. Payne, G. Bojase, M. N. Paddon-Row, M. S. Sherburn, Angew. Chem. 2009, 121, 4930–4933;
10.1002/ange.200901733 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 4836–4839;
- 16bH. Hopf, M. S. Sherburn, Angew. Chem. 2012, 124, 2346–2389;
10.1002/ange.201102987 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 2298–2338;
- 16cH. Hopf, Angew. Chem. 1984, 96, 947–958; Angew. Chem. Int. Ed. Engl. 1984, 23, 948–960.
- 17H. Hopf, G. Maas, Angew. Chem. 1992, 104, 953–977; Angew. Chem. Int. Ed. Engl. 1992, 31, 931–954.
- 18Cyclic [3]dendralenes (trimethylenecyclobutanes) have been studied, but little is known about this class of compounds; see for example: J. K. Williams, W. H. Sharkey, J. Am. Chem. Soc. 1959, 81, 4269–4272; L. Trabert, H. Hopf, D. Schomburg, Chem. Ber. 1981, 114, 2405–2414; W. V. Dower, K. P. C. Vollhardt, Angew. Chem. 1982, 94, 712; Angew. Chem. Int. Ed. Engl. 1982, 21, 1545–1555; W. T. Thorstad, N. S. Mills, D. Q. Buckelew, L. S. Govea, J. Org. Chem. 1989, 54, 713–776.
- 19Spotted onto a TLC plate from CH2Cl2, this fraction is initially orange/brown and slowly turns to green as the solvent evaporates.
- 20F. Bureš, O. Pytela, M. Kivala, F. Diederich, J. Phys. Org. Chem. 2011, 24, 274–281; B. Strehmel, A. M. Sarker, H. Detert, ChemPhysChem 2003, 4, 249–259.
- 21For other rectangular [4]radialenes, see
- 21aA. E. Learned, A. M. Arif, P. J. Stang, J. Org. Chem. 1988, 53, 3122–3123;
- 21bP. I. Dosa, G. D. Whitener, K. P. C. Vollhardt, A. D. Bond, S. J. Teat, Org. Lett. 2002, 4, 2075–2078.
- 22
- 22aM. Iyoda, H. Otani, M. Oda, Y. Kai, Y. Baba, N. Kasai, J. Am. Chem. Soc. 1986, 108, 5371–5372;
- 22bF. P. van Remoortere, F. P. Boer, J. Am. Chem. Soc. 1970, 92, 3355–3360;
- 22cS. Hashmi, K. Polborn, G. Szeimies, Chem. Ber. 1989, 122, 2399–2401.
- 23D. Bouvy, Z. Janousek, H. G. Viehe, B. Tinant, J.-P. Declercq, Tetrahedron Lett. 1993, 34, 1779–1782.
- 24The structural assignment of 16 rests primarily on the mass spectrum as well as its analogous formation to 15 and analogous properties. 1H and 13C NMR spectra were acquired, but show severely broadened signals that limit their usefulness (spectra are supplied in the Supporting Information).
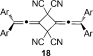
- 25Formation of the head-head dimer 18, cannot be ruled out, but no evidence for supporting formation of 18 has been observed to date.
- 26A somewhat similar addition of TCNE to an electron-rich [3]cumulene has been reported; see N. Islam, M. Tsukayama, Y. Kawamura, Int. J. Mod. Phys. B 2006, 20, 4619–4624.
- 27See, for example:, R. Pal, R. J. Clark, M. Manoharan, I. V. Alabugin, J. Org. Chem. 2010, 75, 8689–8692.