Total Synthesis of the Biscarbazole Alkaloids Murrafoline A–D by a Domino Sonogashira Coupling/Claisen Rearrangement/Electrocyclization Reaction†
Dr. V. Pavan Kumar
Department of Chemistry, Technische Universität Dresden, Bergstrasse 66, 01069 Dresden (Germany) http://www.chm.tu-dresden.de/oc2/
Search for more papers by this authorDr. Konstanze K. Gruner
Department of Chemistry, Technische Universität Dresden, Bergstrasse 66, 01069 Dresden (Germany) http://www.chm.tu-dresden.de/oc2/
Search for more papers by this authorDr. Olga Kataeva
Department of Chemistry, Technische Universität Dresden, Bergstrasse 66, 01069 Dresden (Germany) http://www.chm.tu-dresden.de/oc2/
Search for more papers by this authorCorresponding Author
Prof. Dr. Hans-Joachim Knölker
Department of Chemistry, Technische Universität Dresden, Bergstrasse 66, 01069 Dresden (Germany) http://www.chm.tu-dresden.de/oc2/
Department of Chemistry, Technische Universität Dresden, Bergstrasse 66, 01069 Dresden (Germany) http://www.chm.tu-dresden.de/oc2/Search for more papers by this authorDr. V. Pavan Kumar
Department of Chemistry, Technische Universität Dresden, Bergstrasse 66, 01069 Dresden (Germany) http://www.chm.tu-dresden.de/oc2/
Search for more papers by this authorDr. Konstanze K. Gruner
Department of Chemistry, Technische Universität Dresden, Bergstrasse 66, 01069 Dresden (Germany) http://www.chm.tu-dresden.de/oc2/
Search for more papers by this authorDr. Olga Kataeva
Department of Chemistry, Technische Universität Dresden, Bergstrasse 66, 01069 Dresden (Germany) http://www.chm.tu-dresden.de/oc2/
Search for more papers by this authorCorresponding Author
Prof. Dr. Hans-Joachim Knölker
Department of Chemistry, Technische Universität Dresden, Bergstrasse 66, 01069 Dresden (Germany) http://www.chm.tu-dresden.de/oc2/
Department of Chemistry, Technische Universität Dresden, Bergstrasse 66, 01069 Dresden (Germany) http://www.chm.tu-dresden.de/oc2/Search for more papers by this authorPart 110 of “Transition Metals in Organic Synthesis”; for part 109, see: Ref. 8. We are grateful to the Alexander von Humboldt Foundation (fellowship to V.P.K.) and the Deutsche Forschungsgemeinschaft (grant KN 240/16-1) for financial support. We thank Regina Czerwonka and Nils Richter for experimental support.
Graphical Abstract
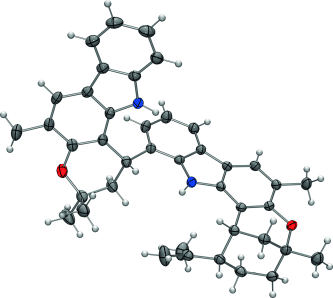
Why take things one step at a time? Aryl–pyran-linked biscarbazole alkaloids of the murrafoline group (see crystal structure of murrafoline A; dark gray: C, red: O, blue: N) were accessed readily by a novel domino reaction sequence involving Sonogashira coupling, a Claisen rearrangement, and electrocyclization. The one-pot procedure enables the straightforward synthesis of these structurally challenging alkaloids in only a few steps.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201305993_sm_miscellaneous_information.pdf4.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aD. P. Chakraborty, S. Roy in Progress in the Chemistry of Organic Natural Products, Vol. 57 (Eds.: ), Springer, Wien, 1991, p. 71;
- 1bD. P. Chakraborty in The Alkaloids, Vol. 44 (Ed.: ), Academic Press, New York, 1993, p. 257;
- 1cH.-J. Knölker, K. R. Reddy, Chem. Rev. 2002, 102, 4303;
- 1dH.-J. Knölker, Curr. Org. Synth. 2004, 1, 309;
- 1eH.-J. Knölker, K. R. Reddy in The Alkaloids, Vol. 65 (Ed.: ), Academic Press, Amsterdam, 2008, p. 1;
10.1016/S1099-4831(07)00001-6 Google Scholar
- 1fA. W. Schmidt, K. R. Reddy, H.-J. Knölker, Chem. Rev. 2012, 112, 3193.
- 2
- 2aH. Furukawa, Trends Heterocycl. Chem. 1993, 3, 185;
- 2bS. Tasler, G. Bringmann, Chem. Rec. 2002, 2, 115.
- 3A. T. McPhail, T.-S. Wu, T. Ohta, H. Furukawa, Tetrahedron Lett. 1983, 24, 5377.
- 4H. Furukawa, T.-S. Wu, C.-S. Kuoh, Chem. Pharm. Bull. 1985, 33, 2611.
- 5H. Furukawa, C. Ito, T.-S. Wu, A. T. McPhail, Chem. Pharm. Bull. 1993, 41, 1249.
- 6H. Furukawa, T.-S. Wu, T. Ohta, C.-S. Kuoh, Chem. Pharm. Bull. 1985, 33, 4132.
- 7
- 7aH.-J. Knölker, C. Hofmann, Tetrahedron Lett. 1996, 37, 7947;
- 7bK. K. Gruner, H.-J. Knölker, Org. Biomol. Chem. 2008, 6, 3902;
- 7cK. K. Gruner, T. Hopfmann, K. Matsumoto, A. Jäger, T. Katsuki, H.-J. Knölker, Org. Biomol. Chem. 2011, 9, 2057.
- 8R. Hesse, K. K. Gruner, O. Kataeva, A. W. Schmidt, H.-J. Knölker, Chem. Eur. J. 2013, DOI: .
- 9C. Börger, O. Kataeva, H.-J. Knölker, Org. Biomol. Chem. 2012, 10, 7269.
- 10
- 10aI. Iwai, J. Ide, Chem. Pharm. Bull. 1962, 10, 926;
- 10bI. Iwai, J. Ide, Chem. Pharm. Bull. 1963, 11, 1042;
- 10cJ. Zsindely, H. Schmid, Helv. Chim. Acta 1968, 51, 1510;
- 10dP. E. Brown, R. A. Lewis, J. Chem. Soc. Perkin Trans. 1 1992, 573.
- 11For reviews, see:
- 11aL. F. Tietze, Chem. Rev. 1996, 96, 115;
- 11bL. F. Tietze, N. Rackelmann, Pure Appl. Chem. 2004, 76, 1967;
- 11cL. F. Tietze, G. Brasche, K. Gericke, Domino Reactions in Organic Synthesis, Wiley-VCH, Weinheim, 2006;
10.1002/9783527609925 Google Scholar
- 11dL. F. Tietze, S. G. Stewart, A. Düfert in Modern Tools for the Synthesis of Complex Bioactive Molecules (Eds.: ), Wiley, Hoboken, 2012, p. 271.
10.1002/9781118342886.ch9 Google Scholar
- 12
- 12aK. Sonogashira, Y. Tohda, N. Hagihara, Tetrahedron Lett. 1975, 16, 4467;
- 12bS. Takahashi, Y. Kuroyama, K. Sonogashira, N. Hagihara, Synthesis 1980, 627;
- 12cE. Negishi, L. Anastasia, Chem. Rev. 2003, 103, 1979;
- 12dR. Chinchilla, C. Nájera, Chem. Rev. 2007, 107, 874.
- 13
- 13aH.-J. Knölker, N. O’Sullivan, Tetrahedron 1994, 50, 10893;
- 13bH.-J. Knölker, K. R. Reddy, Heterocycles 2003, 60, 1049;
- 13cM. P. Krahl, A. Jäger, T. Krause, H.-J. Knölker, Org. Biomol. Chem. 2006, 4, 3215;
- 13dR. Forke, M. P. Krahl, T. Krause, G. Schlechtingen, H.-J. Knölker, Synlett 2007, 268;
- 13eC. Börger, H.-J. Knölker, Synlett 2008, 1698;
- 13fR. Forke, A. Jäger, H.-J. Knölker, Org. Biomol. Chem. 2008, 6, 2481;
- 13gR. Forke, M. P. Krahl, F. Däbritz, A. Jäger, H.-J. Knölker, Synlett 2008, 1870;
- 13hT. Gensch, M. Rönnefahrt, R. Czerwonka, A. Jäger, O. Kataeva, I. Bauer, H.-J. Knölker, Chem. Eur. J. 2012, 18, 770.
- 14For reviews, see:
- 14aH.-J. Knölker, Top. Curr. Chem. 2005, 244, 115;
- 14bH.-J. Knölker, Chem. Lett. 2009, 38, 8;
- 14cR. Forke, K. K. Gruner, K. E. Knott, S. Auschill, S. Agarwal, R. Martin, M. Böhl, S. Richter, G. Tsiavaliaris, R. Fedorov, D. J. Manstein, H. O. Gutzeit, H.-J. Knölker, Pure Appl. Chem. 2010, 82, 1975;
- 14dI. Bauer, H.-J. Knölker, Top. Curr. Chem. 2012, 309, 203.
- 15
- 15aM. D. Charles, P. Schultz, S. L. Buchwald, Org. Lett. 2005, 7, 3965;
- 15bD. S. Surry, S. L. Buchwald, Angew. Chem. 2008, 120, 6438–6461;
10.1002/ange.200800497 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6338–6361.
- 16G. Sartori, G. Casiraghi, L. Bolzoni, G. Casnati, J. Org. Chem. 1979, 44, 803.
- 17Murrafoline A (1): colorless crystals; m.p.: 267 °C (lit.:[3, 5] 260–262 °C); elemental analysis: calcd (%) for C41H42N2O2: C 82.79, H 7.12, N 4.71; found: C 82.40, H 7.97, N 4.32. epi-Murrafoline A (epi-1): colorless crystals; m.p.: 225 °C. Murrafoline B (2): colorless crystals; m.p.: 228 °C (lit.:[4, 5] 234–237 °C); elemental analysis: calcd (%) for C32H30N2O2: C 80.98, H 6.37, N 5.90; found: C 81.00, H 6.74, N 5.70. Murrafoline C (3): colorless crystals, m.p.: 225 °C (lit.:[4, 5] colorless oil); elemental analysis: calcd (%) for C36H34N2O2: C 82.10, H 6.51, N 5.32; found: C 81.38, H 7.14, N 5.41. Murrafoline D (4): colorless crystals; m.p.: 274 °C (lit.:[5] amorphous powder, no m.p. given); elemental analysis: calcd (%) for C32H30N2O2: C 80.98, H 6.37, N 5.90; found: C 80.83, H 6.69, N 5.36. For the 1H NMR (600 MHz) and 13C NMR (150 MHz) spectra of murrafolines A–D (1–4), see the Supporting Information.
- 18Chiral phase: Nucleocel δ-RP (Macherey–Nagel), column dimensions: 250×2.1 mm2, temperature: 20 °C, eluent: MeCN/H2O (85:15), flow rate: 12 mL min−1.
- 19Crystallographic data for 1: C41H42N2O2, M=594.77 g mol−1, crystal size: 0.43×0.26×0.25 mm3, monoclinic, space group P21/c, a=11.880(1), b=12.416(1), c=21.633(2) Å, β=93.801(7)°, V=3183.9(5) Å3, Z=4, ρcalcd=1.241 g cm−3, μ=0.076 mm−1, λ=0.71073 Å, T=198(2) K, θ range: 3.10–27.00°, reflections collected: 32306, independent: 6902 (Rint=0.0516), 412 parameters. The structure was solved by direct methods and refined by full-matrix least-squares on F2; final R indices [I>2σ(I)]: R1=0.0474, wR2=0.1077; maximal residual electron density: 0.199 e Å−3. CCDC 948510 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 20
- 20aH.-J. Knölker, M. Bauermeister, J. Chem. Soc. Chem. Commun. 1990, 664;
- 20bH.-J. Knölker, M. Bauermeister, Tetrahedron 1993, 49, 11221;
- 20cH.-J. Knölker, M. Wolpert, Tetrahedron Lett. 1997, 38, 533;
- 20dH.-J. Knölker, M. Wolpert, Tetrahedron 2003, 59, 5317.
- 21
- 21aM. P. Krahl, PhD thesis, TU Dresden, 2006;
- 21bB. Liégault, D. Lee, M. P. Huestis, D. R. Stuart, K. Fagnou, J. Org. Chem. 2008, 73, 5022;
- 21cC. Börger, M. P. Krahl, M. Gruner, O. Kataeva, H.-J. Knölker, Org. Biomol. Chem. 2012, 10, 5189.
- 22Crystallographic data for 25: C34H32Cl2N2O5, M=619.52 g mol−1, crystal size: 0.38×0.25×0.15 mm3, monoclinic, space group C2/c, a=35.976(3), b=14.475(1), c=13.004(1) Å, V=6520.6(9) Å3, Z=8, ρcalcd=1.262 g cm−3, μ=0.242 mm−1, λ=0.71073 Å, T=198(2) K, θ range: 1.18–26.00°, reflections collected: 63047, independent: 6286 (Rint=0.0963), 446 parameters. The structure was solved by direct methods and refined by full-matrix least-squares on F2; final R indices [I>2σ(I)]: R1=0.0729, wR2=0.02073; maximal residual electron density: 0.765 e Å−3. The crystal contains a void filled with disordered solvent molecules (1719 Å3 per cell unit), which were modeled by dichloromethane and water molecules. CCDC 948511 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.