Trifluoromethanesulfonic Acid Catalyzed Synergetic Oxidative/[3+2] Cyclization of Quinones with Olefins†
Lingkui Meng
College of Chemistry and Molecular Science, Wuhan University, Wuhan 430072 (P.R. China) http://aiwenlei.whu.edu.cn/Main_Website/
Search for more papers by this authorGuanghui Zhang
College of Chemistry and Molecular Science, Wuhan University, Wuhan 430072 (P.R. China) http://aiwenlei.whu.edu.cn/Main_Website/
Search for more papers by this authorDr. Chao Liu
College of Chemistry and Molecular Science, Wuhan University, Wuhan 430072 (P.R. China) http://aiwenlei.whu.edu.cn/Main_Website/
Search for more papers by this authorKun Wu
College of Chemistry and Molecular Science, Wuhan University, Wuhan 430072 (P.R. China) http://aiwenlei.whu.edu.cn/Main_Website/
Search for more papers by this authorCorresponding Author
Prof. Aiwen Lei
College of Chemistry and Molecular Science, Wuhan University, Wuhan 430072 (P.R. China) http://aiwenlei.whu.edu.cn/Main_Website/
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University), Ministry of Education, and Wuhan University School of Pharmaceutical Sciences, Wuhan, 430071 (P.R. China)
College of Chemistry and Molecular Science, Wuhan University, Wuhan 430072 (P.R. China) http://aiwenlei.whu.edu.cn/Main_Website/Search for more papers by this authorLingkui Meng
College of Chemistry and Molecular Science, Wuhan University, Wuhan 430072 (P.R. China) http://aiwenlei.whu.edu.cn/Main_Website/
Search for more papers by this authorGuanghui Zhang
College of Chemistry and Molecular Science, Wuhan University, Wuhan 430072 (P.R. China) http://aiwenlei.whu.edu.cn/Main_Website/
Search for more papers by this authorDr. Chao Liu
College of Chemistry and Molecular Science, Wuhan University, Wuhan 430072 (P.R. China) http://aiwenlei.whu.edu.cn/Main_Website/
Search for more papers by this authorKun Wu
College of Chemistry and Molecular Science, Wuhan University, Wuhan 430072 (P.R. China) http://aiwenlei.whu.edu.cn/Main_Website/
Search for more papers by this authorCorresponding Author
Prof. Aiwen Lei
College of Chemistry and Molecular Science, Wuhan University, Wuhan 430072 (P.R. China) http://aiwenlei.whu.edu.cn/Main_Website/
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University), Ministry of Education, and Wuhan University School of Pharmaceutical Sciences, Wuhan, 430071 (P.R. China)
College of Chemistry and Molecular Science, Wuhan University, Wuhan 430072 (P.R. China) http://aiwenlei.whu.edu.cn/Main_Website/Search for more papers by this authorThis work was supported by the 973 Program (2012CB725302), the National Natural Science Foundation of China (21025206 and 21272180), the Research Fund for the Doctoral Program of Higher Education of China (20120141130002). and the China Postdoctoral Science Foundation funded project (2012M521458). We are also grateful for support from the Program for Changjiang Scholars and Innovative Research Team in University (IRT1030).
Graphical Abstract
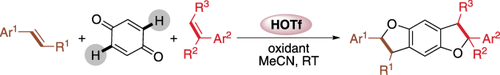
The proton did it! Tetrahydrobenzodifurans have been synthesized through the trifluoromethanesulfonic acid (HOTf) catalyzed direct oxidative CH functionalization of benzoquinone with olefins. A variety of substituents were found to be tolerated, and a synergetic oxidative/[3+2] cyclization mechanism was proposed based on the experimental results.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201305885_sm_miscellaneous_information.pdf2.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aX. Chen, K. M. Engle, D.-H. Wang, J.-Q. Yu, Angew. Chem. 2009, 121, 5196; Angew. Chem. Int. Ed. 2009, 48, 5094;
- 1bA. E. Wendlandt, A. M. Suess, S. S. Stahl, Angew. Chem. 2011, 123, 11256;
10.1002/ange.201103945 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 11062;
- 1cJ. P. Lewtak, D. T. Gryko, Chem. Commun. 2012, 48, 10069;
- 1dC.-L. Sun, B.-J. Li, Z.-J. Shi, Chem. Commun. 2010, 46, 677;
- 1eC. Liu, H. Zhang, W. Shi, A. Lei, Chem. Rev. 2011, 111, 1780;
- 1fB.-J. Li, Z.-J. Shi, Chem. Soc. Rev. 2012, 41, 5588;
- 1gW. Shi, C. Liu, A. Lei, Chem. Soc. Rev. 2011, 40, 2761;
- 1hG. Song, F. Wang, X. Li, Chem. Soc. Rev. 2012, 41, 3651;
- 1iC. Zhang, C. Tang, N. Jiao, Chem. Soc. Rev. 2012, 41, 3464;
- 1jB. Karimi, H. Behzadnia, D. Elhamifar, P. F. Akhavan, F. K. Esfahani, A. Zamani, Synthesis 2010, 1399.
- 2
- 2aC.-L. Sun, H. Li, D.-G. Yu, M. Yu, X. Zhou, X.-Y. Lu, K. Huang, S.-F. Zheng, B.-J. Li, Z.-J. Shi, Nat. Chem. 2010, 2, 1044;
- 2bE. Shirakawa, K.-i. Itoh, T. Higashino, T. Hayashi, J. Am. Chem. Soc. 2010, 132, 15537;
- 2cW. Liu, H. Cao, H. Zhang, H. Zhang, K. H. Chung, C. He, H. Wang, F. Y. Kwong, A. Lei, J. Am. Chem. Soc. 2010, 132, 16737;
- 2dS. Yanagisawa, K. Ueda, T. Taniguchi, K. Itami, Org. Lett. 2008, 10, 4673;
- 2eH. A. Duong, R. E. Gilligan, M. L. Cooke, R. J. Phipps, M. J. Gaunt, Angew. Chem. 2011, 123, 483; Angew. Chem. Int. Ed. 2011, 50, 463;
- 2fL. Chen, E. Shi, Z. Liu, S. Chen, W. Wei, H. Li, K. Xu, X. Wan, Chem. Eur. J. 2011, 17, 4085;
- 2gW. Tu, P. E. Floreancig, Angew. Chem. 2009, 121, 4637; Angew. Chem. Int. Ed. 2009, 48, 4567;
- 2hZ.-Q. Liu, L. Sun, J.-G. Wang, J. Han, Y.-K. Zhao, B. Zhou, Org. Lett. 2009, 11, 1437;
- 2iW. Tu, L. Liu, P. E. Floreancig, Angew. Chem. 2008, 120, 4252; Angew. Chem. Int. Ed. 2008, 47, 4184;
- 2jY. Zhang, C.-J. Li, J. Am. Chem. Soc. 2006, 128, 4242.
- 3
- 3aP. Hu, S. Huang, J. Xu, Z.-J. Shi, W. Su, Angew. Chem. 2011, 123, 10100; Angew. Chem. Int. Ed. 2011, 50, 9926;
- 3bY. Fujiwara, V. Domingo, I. B. Seiple, R. Gianatassio, M. Del Bel, P. S. Baran, J. Am. Chem. Soc. 2011, 133, 3292.
- 4
- 4aS. V. Miert, S. V. Dyck, T. J. Schmidt, R. Brun, A. Vlietinck, G. Lemière, L. Pieters, Bioorg. Med. Chem. 2005, 13, 661;
- 4bG.-H. Chu, M. Gu, J. A. Cassel, S. Belanger, T. M. Graczyk, R. N. DeHaven, N. Conway-James, M. Koblish, P. J. Little, D. L. DeHaven-Hudkins, R. E. Dolle, Bioorg. Med. Chem. Lett. 2005, 15, 5114;
- 4cR.-V. Nguyen, C.-J. Li, Synlett 2008, 1897;
- 4dE. Moreno-Clavijo, A. J. Moreno-Vargas, R. Kieffer, T. Sigstam, A. T. Carmona, I. Robina, Org. Lett. 2011, 13, 6244.
- 5
- 5aP. Fries, D. Halter, A. Kleinschek, J. Hartung, J. Am. Chem. Soc. 2011, 133, 3906;
- 5bA. Taleb, M. Lahrech, S. Hacini, J. Thibonnet, J.-L. Parrain, Synlett 2009, 1597;
- 5cX. Guo, R. Yu, H. Li, Z. Li, J. Am. Chem. Soc. 2009, 131, 17387;
- 5dP. Chen, J. Wang, K. Liu, C. Li, J. Org. Chem. 2008, 73, 339;
- 5eB. M. Trost, A. McClory, Angew. Chem. 2007, 119, 2120;
10.1002/ange.200604183 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 2074;
- 5fA. C. Tadd, M. R. Fielding, M. C. Willis, Tetrahedron Lett. 2007, 48, 7578;
- 5gM. Nagamochi, Y.-Q. Fang, M. Lautens, Org. Lett. 2007, 9, 2955;
- 5hF. Felluga, F. Ghelfi, U. M. Pagnoni, A. F. Parsons, M. Pattarozzi, F. Roncaglia, E. Valentin, Synthesis 2007, 2007, 1882;
- 5iJ. S. Bryans, N. E. A. Chessum, N. Huther, A. F. Parsons, F. Ghelfi, Tetrahedron 2003, 59, 6221.
- 6
- 6aN. Hayashi, Y. Saito, H. Higuchi, K. Suzuki, J. Phys. Chem. A 2009, 113, 5342;
- 6bR. Shukla, S. H. Wadumethrige, S. V. Lindeman, R. Rathore, Org. Lett. 2008, 10, 3587;
- 6cH. Tsuji, C. Mitsui, L. Ilies, Y. Sato, E. Nakamura, J. Am. Chem. Soc. 2007, 129, 11902.
- 7
- 7aZ. Huang, L. Jin, Y. Feng, P. Peng, H. Yi, A. Lei, Angew. Chem. 2013, 125, 7292–7296; Angew. Chem. Int. Ed. 2013, 52, 7151–7155;
- 7bY. Okada, T. Yoshioka, M. Koike, K. Chiba, Tetrahedron Lett. 2011, 52, 4690;
- 7cS. Kim, S. Noda, K. Hayashi, K. Chiba, Org. Lett. 2008, 10, 1827;
- 7dK. Chiba, M. Fukuda, S. Kim, Y. Kitano, M. Tada, J. Org. Chem. 1999, 64, 7654.
- 8
- 8aV. V. Kouznetsov, D. R. Merchan Arenas, A. R. Romero Bohórquez, Tetrahedron Lett. 2008, 49, 3097;
- 8bH. Ohara, H. Kiyokane, T. Itoh, Tetrahedron Lett. 2002, 43, 3041;
- 8cT. A. Engler, K. D. Combrink, M. A. Letavic, K. O. Lynch, J. E. Ray, J. Org. Chem. 1994, 59, 6567;
- 8dT. A. Engler, K. D. Combrink, J. E. Ray, J. Am. Chem. Soc. 1988, 110, 7931;
- 8eJ. S. Yadav, B. V. S. Reddy, G. Kondaji, Synthesis 2003, 1100;
- 8fT. A. Engler, R. Iyengar, J. Org. Chem. 1998, 63, 1929.
- 9K. Matsui, Y. Segawa, T. Namikawa, K. Kamada, K. Itami, Chem. Sci. 2013, 4, 84.
- 10
- 10aC. Liu, S. Tang, D. Liu, J. Yuan, L. Zheng, L. Meng, A. Lei, Angew. Chem. 2012, 124, 3698; Angew. Chem. Int. Ed. 2012, 51, 3638;
- 10bP. S. Engel, H. J. Park, H. Mo, S. Duan, Tetrahedron 2010, 66, 8805.