Intact P4 Tetrahedra as Terminal and Bridging Ligands in Neutral Complexes of Manganese†
Correction(s) for this article
-
Corrigendum: Intact P4 Tetrahedra as Terminal and Bridging Ligands in Neutral Complexes of Manganese
- Sebastian Heinl,
- Eugenia V. Peresypkina,
- Alexey Y. Timoshkin,
- Piero Mastrorilli,
- Vito Gallo,
- Manfred Scheer,
- Volume 53Issue 25Angewandte Chemie International Edition
- pages: 6296-6296
- First Published online: June 12, 2014
M. Sc. Sebastian Heinl
Institute of Inorganic Chemistry, University of Regensburg, 93040 Regensburg (Germany) http://www.ur.de/chemie-pharmazie/anorganische-chemie-scheer/
Search for more papers by this authorDr. Eugenia V. Peresypkina
Institute of Inorganic Chemistry SD RAS, Ak. Lavrentiev prosp. 3, Novosibirsk 630090 (Russia)
Search for more papers by this authorProf. Dr. Alexey Y. Timoshkin
Department of Chemistry, St. Petersburg State University, 198504 St. Petersburg (Russia)
Search for more papers by this authorProf. Dr. Piero Mastrorilli
Dipartimento di Ingegneria Civile, Ambientale, del Territorio, Edile e di Chimica (DICATECh), Politecnico di Bari via Orabona 4, 70125 Bari (Italy)
Search for more papers by this authorDr. Vito Gallo
Dipartimento di Ingegneria Civile, Ambientale, del Territorio, Edile e di Chimica (DICATECh), Politecnico di Bari via Orabona 4, 70125 Bari (Italy)
Search for more papers by this authorCorresponding Author
Prof. Dr. Manfred Scheer
Institute of Inorganic Chemistry, University of Regensburg, 93040 Regensburg (Germany) http://www.ur.de/chemie-pharmazie/anorganische-chemie-scheer/
Institute of Inorganic Chemistry, University of Regensburg, 93040 Regensburg (Germany) http://www.ur.de/chemie-pharmazie/anorganische-chemie-scheer/Search for more papers by this authorM. Sc. Sebastian Heinl
Institute of Inorganic Chemistry, University of Regensburg, 93040 Regensburg (Germany) http://www.ur.de/chemie-pharmazie/anorganische-chemie-scheer/
Search for more papers by this authorDr. Eugenia V. Peresypkina
Institute of Inorganic Chemistry SD RAS, Ak. Lavrentiev prosp. 3, Novosibirsk 630090 (Russia)
Search for more papers by this authorProf. Dr. Alexey Y. Timoshkin
Department of Chemistry, St. Petersburg State University, 198504 St. Petersburg (Russia)
Search for more papers by this authorProf. Dr. Piero Mastrorilli
Dipartimento di Ingegneria Civile, Ambientale, del Territorio, Edile e di Chimica (DICATECh), Politecnico di Bari via Orabona 4, 70125 Bari (Italy)
Search for more papers by this authorDr. Vito Gallo
Dipartimento di Ingegneria Civile, Ambientale, del Territorio, Edile e di Chimica (DICATECh), Politecnico di Bari via Orabona 4, 70125 Bari (Italy)
Search for more papers by this authorCorresponding Author
Prof. Dr. Manfred Scheer
Institute of Inorganic Chemistry, University of Regensburg, 93040 Regensburg (Germany) http://www.ur.de/chemie-pharmazie/anorganische-chemie-scheer/
Institute of Inorganic Chemistry, University of Regensburg, 93040 Regensburg (Germany) http://www.ur.de/chemie-pharmazie/anorganische-chemie-scheer/Search for more papers by this authorThis work was supported by the Deutsche Forschungsgemeinschaft. S.H. thanks the Fonds der Chemischen Industrie for a PhD scholarship. We are grateful to COST action CM0802 (PhoSciNet) for general support.
Graphical Abstract
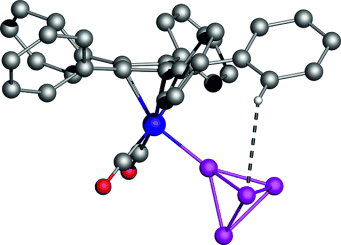
Static dynamic: Neutral, end-on bound white-phosphorus complexes with unprecedented stability in the solid state and solution were synthesized (see structure: gray C, blue Mn, red O, violet P). While the C5(4-nBuC6H4)5 ligands are stationary at low temperature on the NMR timescale, the P4 ligands rotate rapidly. Despite the unfavorable γP/γH ratio, a heteronuclear Overhauser effect between the protons in ortho position and the basal P atoms was detected.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201305315_sm_miscellaneous_information.pdf639.1 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. Scheer, G. Balazs, A. Seitz, Chem. Rev. 2010, 110, 4236–4256.
- 2D. Tofan, C. C. Cummins, Angew. Chem. 2010, 122, 7678–7680;
10.1002/ange.201004385 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7516–7518; For the reaction of red phosphorus with alkenes in alkaline media:
- 2aA. V. Artem’ev, S. F. Malysheva, A. O. Korocheva, I. Y. Bagryanskaya, Heteroat. Chem. 2012, 23, 568–573;
- 2bS. F. Malysheva, A. O. Korocheva, N. A. Belogorlova, A. V. Artem’ev, N. K. Gusarova, B. A. Trofimov, Dokl. Chem. 2012, 445, 164–165;
- 2cS. F. Malysheva, V. A. Kuimov, A. V. Artem’ev, N. A. Belogorlova, A. I. Albanov, N. K. Gusarova, B. A. Trofimov, Russ. Chem. Bull. 2012, 61, 1787–1791.
- 3
- 3aB. M. Cossairt, N. A. Piro, C. C. Cummins, Chem. Rev. 2010, 110, 4164–4177;
- 3bM. Caporali, L. Gonsalvi, A. Rossin, M. Peruzzini, Chem. Rev. 2010, 110, 4178–4235.
- 4
- 4aP. Dapporto, S. Midollini, L. Sacconi, Angew. Chem. 1979, 91, 510; Angew. Chem. Int. Ed. Engl. 1979, 18, 469–470;
- 4bP. Dapporto, L. Sacconi, P. Stoppioni, F. Zanobini, Inorg. Chem. 1981, 20, 3834–3839.
- 5T. Gröer, G. Baum, M. Scheer, Organometallics 1998, 17, 5916–5919.
- 6
- 6aI. de los Rios, J.-R. Hamon, P. Hamon, C. Lapinte, L. Toupet, A. Romerosa, M. Peruzzini, Angew. Chem. 2001, 113, 4028–4030;
10.1002/1521-3757(20011015)113:20<4028::AID-ANGE4028>3.0.CO;2-O Google ScholarAngew. Chem. Int. Ed. 2001, 40, 3910–3912;10.1002/1521-3773(20011015)40:20<3910::AID-ANIE3910>3.0.CO;2-2 CAS PubMed Web of Science® Google Scholar
- 6bM. Di Vaira, P. Frediani, S. S. Costantini, M. Peruzzini, P. Stoppioni, Dalton Trans. 2005, 2234–2236;
- 6cM. Di Vaira, M. Peruzzini, S. S. Costantini, P. Stoppioni, J. Organomet. Chem. 2006, 691, 3931–3937;
- 6dP. Barbaro, V. M. Di, M. Peruzzini, S. S. Costantini, P. Stoppioni, Chem. Eur. J. 2007, 13, 6682–6690;
- 6eM. Caporali, M. Di Vaira, M. Peruzzini, S. S. Costantini, P. Stoppioni, F. Zanobini, Eur. J. Inorg. Chem. 2010, 152–158;
- 6fM. Di Vaira, M. Peruzzini, S. S. Costantini, P. Stoppioni, J. Organomet. Chem. 2010, 695, 816–820;
- 6gM. Peruzzini, L. Marvelli, A. Romerosa, R. Rossi, F. Vizza, F. Zanobini, Eur. J. Inorg. Chem. 1999, 931–933;
10.1002/(SICI)1099-0682(199906)1999:6<931::AID-EJIC931>3.0.CO;2-1 CAS Web of Science® Google Scholar
- 6hV. Mirabello, M. Caporali, V. Gallo, L. Gonsalvi, A. Ienco, M. Latronico, P. Mastrorilli, M. Peruzzini, Dalton Trans. 2011, 40, 9668–9671;
- 6iV. Mirabello, M. Caporali, V. Gallo, L. Gonsalvi, D. Gudat, W. Frey, A. Ienco, M. Latronico, P. Mastrorilli, M. Peruzzini, Chem. Eur. J. 2012, 18, 11238–11250.
- 7There is one neutral example described [Cl(PPh3)2Ru(μ-Cl)3Ru(PPh3)2(η1-P4)] revealing high Lewis acidity, as a result of the numerous Cl ligands cf.: M. Peruzzini, S. Manas, A. Romerosa, A. Vacca, Mendeleev Commun. 2000, 10, 134–135.
10.1070/MC2000v010n04ABEH001271 Google Scholar
- 8
- 8aM. Scheer, E. Herrmann, Z. Chem. 1990, 30, 41–55;
- 8bO. J. Scherer, Acc. Chem. Res. 1999, 32, 751–762.
- 9L. D. Field, T. He, P. Humphrey, A. F. Masters, P. Turner, Polyhedron 2006, 25, 1498–1506.
- 10See Supporting Information for details on NMR spectroscopy and computations.
- 11“Transition Metal Coordination Chemistry”: J. Okuda in Topics in Current Chemistry, Vol. 160 (Ed.: ), Springer, Heidelberg, 1992, pp. 97–145.
- 12M. Latronico, F. Polini, V. Gallo, P. Mastrorilli, B. Calmuschi-Cula, U. Englert, N. Re, T. Repo, M. Räisänen, Inorg. Chem. 2008, 47, 9779–9796.
- 13At a constant internuclear distance in H–X pairs, the heteronuclear dipolar coupling decreases proportionally to coefficient k, calculated by the Equation k=0.44(νX/νH)2IX(IX+1) where νX and νH are the NMR frequencies of X and H nuclei, respectively, and IX is the spin of X nuclei: V. I. Bakhmutov, Practical NMR relaxation for chemists, Wiley, Chichester, 2004.
10.1002/0470094486 Google Scholar
- 14On the basis of the interatomic distances observed in the solid state, a correlation between the apical P atom of the cage and the ortho H atoms of the CpBIG phenyl groups should be also expected to be seen in the 31P-1H HOESY spectrum of a [D8]toluene solution of 2 at 273 K. However, the corresponding cross peak is missing, most likely because of to the lower intensity of the signal of the apical P atom.
- 15CCDC 943753 (2) and 943754 (3) contain the detailed crystallographic information for this manuscript. The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif..