Cobalt-Catalyzed Intramolecular Olefin Hydroarylation Leading to Dihydropyrroloindoles and Tetrahydropyridoindoles†
Zhenhua Ding
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore) http://www3.ntu.edu.sg/home/nyoshikai/yoshikai_group/Home.html
Search for more papers by this authorCorresponding Author
Prof. Naohiko Yoshikai
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore) http://www3.ntu.edu.sg/home/nyoshikai/yoshikai_group/Home.html
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore) http://www3.ntu.edu.sg/home/nyoshikai/yoshikai_group/Home.htmlSearch for more papers by this authorZhenhua Ding
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore) http://www3.ntu.edu.sg/home/nyoshikai/yoshikai_group/Home.html
Search for more papers by this authorCorresponding Author
Prof. Naohiko Yoshikai
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore) http://www3.ntu.edu.sg/home/nyoshikai/yoshikai_group/Home.html
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore) http://www3.ntu.edu.sg/home/nyoshikai/yoshikai_group/Home.htmlSearch for more papers by this authorThis work was supported by the National Research Foundation Singapore (NRF-RF2009-05), Nanyang Technological University, and JST, CREST. We thank Dr. Yongxin Li and Dr. Rakesh Ganguly (Nanyang Technological University) for assistance in X-ray diffraction analysis.
Graphical Abstract
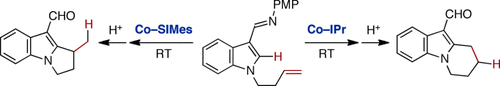
Regiodivergent catalysis: Cobalt N-heterocyclic carbene (NHC) catalysts promote intramolecular olefin hydroarylation of indoles bearing an N-homoallyl or bis(homoallyl) tether and a C3 aldimine directing group to afford dihydropyrroloindoles and tetrahydropyridoindoles under mild conditions. The course of the cyclization is dependent on the tether, but can be controlled by the NHC ligand.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201305151_sm_miscellaneous_information.pdf6.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. A. Bit, P. D. Davis, L. H. Elliott, W. Harris, C. H. Hill, E. Keech, H. Kumar, G. Lawton, A. Maw, J. S. Nixon, D. R. Vesey, J. Wadsworth, S. E. Wilkinson, J. Med. Chem. 1993, 36, 21;
- 1bF. G. Gervais, J. P. Morello, C. Beaulieu, N. Sawyer, D. Denis, G. Greig, A. D. Malebranche, G. P. O’Neill, Mol. Pharmacol. 2005, 67, 1834;
- 1cC. Molinaro, P. G. Bulger, E. E. Lee, B. Kosjek, S. Lau, D. Gauvreau, M. E. Howard, D. J. Wallace, P. D. O’Shea, J. Org. Chem. 2012, 77, 2299.
- 2R. M. Wilson, R. K. Thalji, R. G. Bergman, J. A. Ellman, Org. Lett. 2006, 8, 1745.
- 3For reviews, see:
- 3aD. A. Colby, R. G. Bergman, J. A. Ellman, Chem. Rev. 2010, 110, 624;
- 3bF. Kakiuchi, T. Kochi, Synthesis 2008, 3013.
- 4For imine-directed ortho-alkylation, see:
- 4aR. K. Thalji, K. A. Ahrendt, R. G. Bergman, J. A. Ellman, J. Am. Chem. Soc. 2001, 123, 9692;
- 4bR. K. Thalji, J. A. Ellman, R. G. Bergman, J. Am. Chem. Soc. 2004, 126, 7192;
- 4cR. K. Thalji, K. A. Ahrendt, R. G. Bergman, J. A. Ellman, J. Org. Chem. 2005, 70, 6775;
- 4dH. Harada, R. K. Thalji, R. G. Bergman, J. A. Ellman, J. Org. Chem. 2008, 73, 6772.
- 5For C2 alkylation of (benz)imidazoles, see:
- 5aK. L. Tan, R. G. Bergman, J. A. Ellman, J. Am. Chem. Soc. 2001, 123, 2685;
- 5bK. L. Tan, A. Vasudevan, R. G. Bergman, J. A. Ellman, A. J. Souers, Org. Lett. 2003, 5, 2131.
- 6For C2 alkylation of pyridines, see: S. Yotphan, R. G. Bergman, J. A. Ellman, Org. Lett. 2010, 12, 2978.
- 7
- 7aK. A. Ahrendt, R. G. Bergman, J. A. Ellman, Org. Lett. 2003, 5, 1301;
- 7bS. J. O’Malley, K. L. Tan, A. Watzke, R. G. Bergman, J. A. Ellman, J. Am. Chem. Soc. 2005, 127, 13496;
- 7cJ. C. Rech, M. Yato, D. Duckett, B. Ember, P. V. LoGrasso, R. G. Bergman, J. A. Ellman, J. Am. Chem. Soc. 2007, 129, 490;
- 7dA. S. Tsai, R. G. Bergman, J. A. Ellman, J. Am. Chem. Soc. 2008, 130, 6316.
- 8
- 8aN. Fujii, F. Kakiuchi, N. Chatani, S. Murai, Chem. Lett. 1996, 939;
- 8bN. Fujii, F. Kakiuchi, A. Yamada, N. Chatani, S. Murai, Bull. Chem. Soc. Jpn. 1998, 71, 285;
- 8cN. Fujii, F. Kakiuchi, A. Yamada, N. Chatani, S. Murai, Chem. Lett. 1997, 425.
- 9A bishomoallyl tether was successfully used in Ni-catalyzed intramolecular cyclization of a pyridone derivative: Y. Nakao, H. Idei, K. S. Kanyiva, T. Hiyama, J. Am. Chem. Soc. 2009, 131, 15996.
- 10
- 10aK. Gao, N. Yoshikai, J. Am. Chem. Soc. 2011, 133, 400;
- 10bK. Gao, N. Yoshikai, Angew. Chem. 2011, 123, 7020; Angew. Chem. Int. Ed. 2011, 50, 6888;
- 10cZ. Ding, N. Yoshikai, Beilstein J. Org. Chem. 2012, 8, 1536;
- 10dP.-S. Lee, N. Yoshikai, Angew. Chem. 2013, 125, 1278; Angew. Chem. Int. Ed. 2013, 52, 1240.
- 11L. Ilies, Q. Chen, X. Zeng, E. Nakamura, J. Am. Chem. Soc. 2011, 133, 5221.
- 12An N-benzyl analogue of 1 a also reacted sluggishly under rhodium catalysis. Thus, the [RhCl(PPh3)3] and [{RhCl(coe)2}2]/PCy3 catalytic systems afforded a mixture of 2 a and 3 a in 28 % (1:1.3) and 10 % (1:1.5) yields, respectively.
- 13The structure of 2 i was unambiguously determined by X-ray diffraction analysis. Upon being left in hexane/EtOAc, the aldehyde 3 l was oxidized to a carboxylic acid, the structure of which was also determined by X-ray diffraction analysis (see the Supporting Information). CCDC 943911 (2 i) and 943912 (the carboxylic acid derived from 3 l) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 14A. Modak, A. Deb, T. Patra, S. Rana, S. Maity, D. Maiti, Chem. Commun. 2012, 48, 4253.
- 15C.-H. Jun, C. W. Moon, J.-B. Hong, S.-G. Lim, K.-Y. Chung, Y.-H. Kim, Chem. Eur. J. 2002, 8, 485.
10.1002/1521-3765(20020118)8:2<485::AID-CHEM485>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 16While insertion of the olefin moiety into the CoC bond cannot be excluded a priori, we prefer the proposed insertion pathways, which allow the cobalt center to retain the five-membered chelate structure.
- 17For representative reviews on cobalt-catalyzed CC bond formations, see:
- 17aG. Cahiez, A. Moyeux, Chem. Rev. 2010, 110, 1435;
- 17bW. Hess, J. Treutwein, G. Hilt, Synthesis 2008, 3537;
- 17cC. Gosmini, J.-M. Begouin, A. Moncomble, Chem. Commun. 2008, 3221.
- 18J. Mahatthananchai, A. M. Dumas, J. W. Bode, Angew. Chem. 2012, 124, 11114;
10.1002/ange.201201787 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 10954.
- 19For selected examples:
- 19aH. A. Malik, G. J. Sormunen, J. Montgomery, J. Am. Chem. Soc. 2010, 132, 6304;
- 19bA.-R. Shareef, D. H. Sherman, J. Montgomery, Chem. Sci. 2012, 3, 892;
- 19cM. Arndt, M. Dindaroğlu, H.-G. Schmalz, G. Hilt, Org. Lett. 2011, 13, 6236.