Insertion of CS2 into Iridium–Fluorine Bonds†
Paul Kläring
Humboldt-Universität zu Berlin, Department of Chemistry, Brook-Taylor-Strasse 2, 12489 Berlin (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Thomas Braun
Humboldt-Universität zu Berlin, Department of Chemistry, Brook-Taylor-Strasse 2, 12489 Berlin (Germany)
Humboldt-Universität zu Berlin, Department of Chemistry, Brook-Taylor-Strasse 2, 12489 Berlin (Germany)Search for more papers by this authorPaul Kläring
Humboldt-Universität zu Berlin, Department of Chemistry, Brook-Taylor-Strasse 2, 12489 Berlin (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Thomas Braun
Humboldt-Universität zu Berlin, Department of Chemistry, Brook-Taylor-Strasse 2, 12489 Berlin (Germany)
Humboldt-Universität zu Berlin, Department of Chemistry, Brook-Taylor-Strasse 2, 12489 Berlin (Germany)Search for more papers by this authorWe gratefully acknowledge support from the research training group “Fluorine as a Key Element” funded by the Deutsche Forschungsgemeinschaft. We would like to thank L. Zámostná and T. Ahrens for experimental assistance and Dr. C. Müller (Free University Berlin) for helpful discussions concerning the DFT calculations.
Graphical Abstract
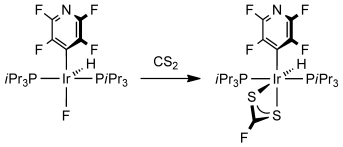
CS2 jumps in: CF bond formation occurs by reactions of the fluorido complexes trans-[Ir(ArF)(F)(H)(PiPr3)2] (ArF=4-C5NF4; see scheme) with CS2 to form the fluorodithiocarbonato species trans-[Ir(ArF)(H)(κ2-(S,S)-S2CF)(PiPr3)2]. DFT studies suggest an unprecedented concerted metathesis-like mechanism for the CF bond-formation step in which CS2 inserts into the IrF bond.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201305106_sm_miscellaneous_information.pdf227.2 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews on fluorinated materials, see:
- 1aJ. Scheirs, Modern Fluoropolymers (Ed.: ), Wiley, New York, 1997;
- 1bK. Johns, G. Stead, J. Fluorine Chem. 2000, 104, 5–18;
- 1cT. Hiyama, Organofluorine Compounds. Chemistry and Applications, Springer, Berlin, 2000;
10.1007/978-3-662-04164-2 Google Scholar
- 1dB. Ameduri, B. Boutevin, Well-Architectured Fluoropolymers: Synthesis Properties and Applications, Elsevier, Amsterdam, 2004;
- 1eP. Kirsch, Modern Fluoroorganic Chemistry. Synthesis Reactivity, Applications, Wiley-VCH, Weinheim, 2004;
10.1002/352760393X Google Scholar
- 1fJ. A. Gladysz, Handbook of Fluorous Chemistry, Wiley-VCH, Weinheim, 2004;
10.1002/3527603905 Google Scholar
- 1g Fluorinated Materials for Energy Conversion, (Eds.: ), Elsevier, Amsterdam, 2005;
- 1hK. Reichenbächer, H. I. Süss, J. Hulliger, Chem. Soc. Rev. 2005, 34, 22–30;
- 1iR. Berger, G. Resnati, P. Metrangolo, E. Weber, J. Hulliger, Chem. Soc. Rev. 2011, 40, 3496–3508;
- 1jP. Metrangolo, F. Meyer, T. Pilati, G. Resnati, G. Terraneo, Angew. Chem. 2008, 120, 6206–6220;
10.1002/ange.200800128 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6114–6127.
- 2For reviews on medicinal aspects of fluorine chemistry, see:
- 2aC. Isanbor, D. O’Hagan, J. Fluorine Chem. 2006, 127, 303–319;
- 2bK. L. Kirk, J. Fluorine Chem. 2006, 127, 1013–1029;
- 2cK. Müller, C. Faeh, F. Diederich, Science 2007, 317, 1881–1886;
- 2dJ.-P. Bégué, D. Bonnet-Delpon, Bioorganic and Medicinal Chemistry of Fluorine, Wiley, Hoboken, 2008;
10.1002/9780470281895 Google Scholar
- 2eS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320–330;
- 2fD. O’Hagan, J. Fluorine Chem. 2010, 131, 1071–1081.
- 3
- 3a Fluorine in Agriculture, 9–11 January 1995, Conference Papers (Ed.: ), Fluorine Technology Ltd., Cheshire, 1994;
- 3bP. Jeschke, ChemBioChem 2004, 5, 570–589.
- 4For examples of applications of [18F] in PET, see:
- 4aM. E. Phelps, Proc. Natl. Acad. Sci. USA 2000, 97, 9226–9233;
- 4bM. J. Adam, D. S. Wilbur, Chem. Soc. Rev. 2005, 34, 153–163;
- 4cS. M. Ametamey, M. Honer, P. A. Schubiger, Chem. Rev. 2008, 108, 1501–1516;
- 4dP. W. Miller, N. J. Long, R. Vilar, A. D. Gee, Angew. Chem. 2008, 120, 9136–9172;
10.1002/ange.200800222 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 8998–9033;
- 4eE. Lee, A. S. Kamlet, D. C. Powers, C. N. Neumann, G. B. Boursalian, T. Furuya, D. C. Choi, J. M. Hooker, T. Ritter, Science 2011, 334, 639–642;
- 4fM. Tredwell, V. Gouverneur, Angew. Chem. 2012, 124, 11590–11602;
10.1002/ange.201204687 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 11426–11437;
- 4gE. Lee, J. M. Hooker, T. Ritter, J. Am. Chem. Soc. 2012, 134, 17456–17458;
- 4hI. S. R. Stenhagen, A. K. Kirjavainen, S. J. Forsback, C. G. Jørgensen, E. G. Robins, S. K. Luthra, O. Solin, V. Gouverneur, Chem. Commun. 2013, 49, 1386–1388.
- 5For reviews on transition-metal-mediated fluorination, see:
- 5aV. V. Grushin, Chem. Eur. J. 2002, 8, 1006–1014;
10.1002/1521-3765(20020301)8:5<1006::AID-CHEM1006>3.0.CO;2-M CAS PubMed Web of Science® Google Scholar
- 5bC. Bobbio, V. Gouverneur, Org. Biomol. Chem. 2006, 4, 2065–2075;
- 5cJ. M. Brown, V. Gouverneur, Angew. Chem. 2009, 121, 8762–8766; Angew. Chem. Int. Ed. 2009, 48, 8610–8614;
- 5dV. Gouverneur, Science 2009, 325, 1630–1630;
- 5eT. Furuya, J. E. M. N. Klein, T. Ritter, Synthesis 2010, 1804–1821;
- 5fT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147–1169;
- 5gT. Furuya, A. S. Kamlet, T. Ritter, Nature 2011, 473, 470–477;
- 5hC. Hollingworth, V. Gouverneur, Chem. Commun. 2012, 48, 2929–2942;
- 5iT. Liang, C. N. Neumann, T. Ritter, Angew. Chem. 2013, 125, 8372–8423; Angew. Chem. Int. Ed. 2013, 32, 8214–8264.
- 6
- 6aL. Hintermann, A. Togni, Angew. Chem. 2000, 112, 4530–4533;
10.1002/1521-3757(20001201)112:23<4530::AID-ANGE4530>3.0.CO;2-D Google ScholarAngew. Chem. Int. Ed. 2000, 39, 4359–4362;10.1002/1521-3773(20001201)39:23<4359::AID-ANIE4359>3.0.CO;2-P CAS PubMed Web of Science® Google Scholar
- 6bA. Togni, A. Mezzetti, P. Barthazy, C. Becker, I. Devillers, R. Frantz, L. Hintermann, M. Perseghini, M. Sanna, Chimia 2001, 55, 801–805;
- 6cP. Barthazy, A. Togni, A. Mezzetti, Organometallics 2001, 20, 3472–3477;
- 6dY. Hamashima, K. Yagi, H. Takano, L. Tamáz, M. Sodeoka, J. Am. Chem. Soc. 2002, 124, 14530–14531;
- 6eY. Hamashima, H. Takano, D. Hotta, M. Sodeoka, Org. Lett. 2003, 5, 3225–3228;
- 6fY. Hamashima, T. Suzuki, H. Takano, Y. Shimura, M. Sodeoka, J. Am. Chem. Soc. 2005, 127, 10164–10165;
- 6gK. L. Hull, W. Q. Anani, M. S. Sanford, J. Am. Chem. Soc. 2006, 128, 7134–7135;
- 6hT. Suzuki, Y. Hamashima, M. Sodeoka, Angew. Chem. 2007, 119, 5531–5535; Angew. Chem. Int. Ed. 2007, 46, 5435–5439;
- 6iT. Suzuki, T. Goto, Y. Hamashira, M. Sodeoka, J. Org. Chem. 2007, 72, 246–250;
- 6jB. C. Gorske, C. T. Mbofana, S. J. Miller, Org. Lett. 2009, 11, 4318–4321;
- 6kA. Hazari, V. Gouverneur, J. M. Brown, Angew. Chem. 2009, 121, 1322–1325;
10.1002/ange.200804310 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 1296–1299;
- 6lW. Wang, J. Jasinski, G. B. Hammond, B. Xu, Angew. Chem. 2010, 122, 7405–7410; Angew. Chem. Int. Ed. 2010, 49, 7247–7252;
- 6mS. Qiu, T. Xu, J. Zhou, Y. Guo, G. Liu, J. Am. Chem. Soc. 2010, 132, 2856–2857;
- 6nP. Tang, T. Furuya, T. Ritter, J. Am. Chem. Soc. 2010, 132, 12150–12154;
- 6oM. H. Katcher, A. G. Doyle, J. Am. Chem. Soc. 2010, 132, 17402–17404;
- 6pC. Hollingworth, A. Hazari, M. N. Hopkinson, M. Tredwell, E. Benedetto, M. Huiban, A. D. Gee, J. M. Brown, V. Gouverneur, Angew. Chem. 2011, 123, 2661–2665; Angew. Chem. Int. Ed. 2011, 50, 2613–2617;
- 6qM. H. Katcher, A. Sha, A. G. Doyle, J. Am. Chem. Soc. 2011, 133, 15902–15905;
- 6rW. Liu, X. Huang, M.-J. Cheng, R. J. Nielsen, W. A. Goddard III, J. T. Groves, Science 2012, 337, 1322–1325;
- 6sP. S. Fier, J. Luo, J. F. Hartwig, J. Am. Chem. Soc. 2013, 135, 2552–2559;
- 6tM.-G. Braun, M. H. Katcher, A. G. Doyle, Chem. Sci. 2013, 4, 1216–1220.
- 7
- 7aA. J. Blake, R. W. Cockman, E. A. V. Ebsworth, J. H. Holloway, J. Chem. Soc. Chem. Commun. 1988, 529–530;
- 7bE. A. V. Ebsworth, N. Robertson, L. J. Yellowlees, J. Chem. Soc. Dalton Trans. 1993, 1031–1037;
- 7cS. A. Brewer, K. S. Coleman, J. Fawcett, J. H. Holloway, E. G. Hope, D. R. Russell, P. G. Watson, J. Chem. Soc. Dalton Trans. 1995, 1073–1076;
- 7dJ. E. Veltheer, P. Burger, R. G. Bergman, J. Am. Chem. Soc. 1995, 117, 12478–12488;
- 7eP. Barthazy, L. Hintermann, R. M. Stoop, M. Wörle, A. Mezzetti, A. Togni, Helv. Chim. Acta 1999, 82, 2448–2453;
10.1002/(SICI)1522-2675(19991215)82:12<2448::AID-HLCA2448>3.0.CO;2-G CAS Web of Science® Google Scholar
- 7fP. Barthazy, R. M. Stoop, M. Wörle, A. Togni, A. Mezzetti, Organometallics 2000, 19, 2844–2852;
- 7gL. Hintermann, F. Läng, P. Maire, A. Togni, Eur. J. Inorg. Chem. 2006, 1396–1412;
- 7hT. Furuya, H. M. Kaiser, T. Ritter, Angew. Chem. 2008, 120, 6082–6085;
10.1002/ange.200802164 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5993–5996;
- 7iT. Furuya, T. Ritter, J. Am. Chem. Soc. 2008, 130, 10060–10061;
- 7jN. D. Ball, M. S. Sanford, J. Am. Chem. Soc. 2009, 131, 3796–3797;
- 7kT. Furuya, A. E. Strom, T. Ritter, J. Am. Chem. Soc. 2009, 131, 1662–1663;
- 7lT. Furuya, T. Ritter, Org. Lett. 2009, 11, 2860–2863;
- 7mP. Tang, T. Ritter, Tetrahedron 2011, 67, 4449–4454;
- 7nD. Breyer, T. Braun, P. Kläring, Organometallics 2012, 31, 1417–1424.
- 8
- 8aW. J. Marshall, V. V. Grushin, Organometallics 2003, 22, 555–562;
- 8bV. V. Grushin, W. J. Marshall, J. Am. Chem. Soc. 2004, 126, 3068–3069;
- 8cW. J. Marshall, V. V. Grushin, Organometallics 2004, 23, 3343–3347;
- 8dS. A. Macgregor, D. C. Roe, W. J. Marshall, K. M. Bloch, V. I. Bakhmutov, V. V. Grushin, J. Am. Chem. Soc. 2005, 127, 15304–15321;
- 8eV. V. Grushin, W. J. Marshall, Organometallics 2007, 26, 4997–5002;
- 8fD. V. Yandulov, N. G. Tran, J. Am. Chem. Soc. 2007, 129, 1342–1358;
- 8gD. A. Watson, M. Su, G. Teverovkiy, Y. Zhang, J. Garzía-Fortanet, T. Kinzel, S. L. Buchwald, Science 2009, 325, 1661–1664;
- 8hT. Furuya, D. Benitez, E. Tkatchouk, A. E. Strom, P. Tang, W. A. Goddard III, T. Ritter, J. Am. Chem. Soc. 2010, 132, 3793–3807.
- 9J. A. Akana, K. X. Bhattacharyya, P. Müller, J. P. Sadighi, J. Am. Chem. Soc. 2007, 129, 7736–7737.
- 10We denote the S2CF−anion as fluorodithiocarbonate anion and the corresponding ligand fluorodithiocarbonato ligand in accordance to:
- 10a Nomenclature of Inorganic Chemistry, IUPAC Recommendations 1990 (Ed.: ), Blackwell Scientific Publications, Oxford, 1990, pp. 122–142;
- 10bX. Zhang, U. Gross, K. Seppelt, Angew. Chem. 1995, 107, 2019–2021; Angew. Chem. Int. Ed. Engl. 1995, 34, 1858–1860;
- 10c Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005 (Eds.: ), RSC Publishing, Cambridge, 2005, pp. 124–141.
- 11J. A. Evans, M. J. Hacker, R. D. W. Kemmitt, D. R. Russell, J. Stocks, J. Chem. Soc. Chem. Commun. 1972, 72–73.
- 12For F⋅⋅⋅F separations in bifluoride ions, see:
- 12aD. Lentz, K. Seppelt, U. Rüdiger, J. Fluorine Chem. 1997, 84, 103–106;
- 12bS. Y. Troyanov, I. V. Morozov, E. Kemnitz, Z. Anorg. Allg. Chem. 2005, 631, 1651–1654, and references herein;
- 12cT. Braun, A. Steffen, V. Schorlemer, B. Neumann, H.-G. Stammler, Dalton Trans. 2005, 3331–3335;
- 12dP. Tang, W. Wang, T. Ritter, J. Am. Chem. Soc. 2011, 133, 11482–11484.
- 13P. Kläring, A.-K. Jungton, T. Braun, C. Müller, Eur. J. Inorg. Chem. 2012, 1430–1436.
- 14
- 14aM. Ahijado, T. Braun, D. Noveski, N. Kocher, B. Neumann, D. Stalke, H.-G. Stammler, Angew. Chem. 2005, 117, 7107–7111;
10.1002/ange.200501615 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 6947–6951;
- 14bM. Ahijado Salomon, T. Braun, I. Krossing, Dalton Trans. 2008, 5197–5206;
- 14cM. Ahijado Salomon, A.-K. Jungton, T. Braun, Dalton Trans. 2009, 7669–7677;
- 14dG. Meier, T. Braun, Angew. Chem. 2012, 124, 12732–12737;
10.1002/ange.201207073 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 12564–12569.
- 15P. Kläring, S. Pahl, A. Penner, T. Braun, Dalton Trans. 2011, 40, 6785–6791.
- 16See the Supporting Information for computational details and a full reference for the Gaussian 09 programme package: Gaussian 09, Revision A.02, M. J. Frisch et al., Gaussian Inc., Wallingford CT, 2009.
- 17
- 17aN. Kuhn, H. Bohnen, J. Fahl, D. Blaser, R. Boese, Chem. Ber. 1996, 129, 1579–1585;
- 17bT. Böttcher, B. S. Bassil, G.-V. Röschenthaler, Inorg. Chem. 2012, 51, 763–765.
- 18For experimentally determined HF separations in solid, liquid, and gaseous hydrogen fluoride, see:
- 18aM. Atoji, W. Lipscomp, Acta Crystallogr. 1954, 7, 173–175;
- 18bM. Deraman, J. C. Core, J. G. Powles, J. H. Holloway, P. Chieux, Mol. Phys. 1985, 55, 1351–1367;
- 18cT. Pfleiderer, I. Waldner, H. Bertagnolli, K. Tödheide, H. E. Fischer, J. Chem. Phys. 2000, 113, 3690–3696;
- 18dS. E. McLain, C. J. Benmore, J. E. Siewenie, J. Urquidi, J. F. C. Turner, Angew. Chem. 2004, 116, 1986–1989;
10.1002/ange.200353289 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 1952–1955;
- 18eM. Kreitmeir, G. Heusel, H. Bertagnolli, K. Tödheide, C. J. Mundy, J. Chem. Phys. 2005, 122, 154511–8.
- 19
- 19aL. A. Watson, D. V. Yandulov, K. G. Caulton, J. Am. Chem. Soc. 2001, 123, 603–611;
- 19bS. P. Reade, M. F. Mahon, M. K. Whittlesey, J. Am. Chem. Soc. 2009, 131, 1847–1861;
- 19cJ. A. Panetier, S. A. Macgregor, M. K. Whittlesey, Angew. Chem. 2011, 123, 2835–2838; Angew. Chem. Int. Ed. 2011, 50, 2783–2786;
- 19dA. Nova, R. Mas-Ballesté, A. Lledós, Organometallics 2012, 31, 1245–1256, and references herein;
- 19eJ.-P. Werner, P. Burger, J. Phys. Chem. A 2011, 115, 13885–13895;
- 19fD. Lentz, T. Braun, M. F. Kuehnel, Angew. Chem. 2013, 125, 5328–5332;
10.1002/ange.201209731 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 3328–3348;
- 19gM. F. Kühnel, P. Holstein, M. Kliche, J. Krüger, S. Matthies, D. Nitsch, J. Schutt, M. Sparenberg, D. Lentz, Chem. Eur. J. 2012, 18, 10701–10714;
- 19hM. F. Kühnel, D. Lentz, Angew. Chem. 2010, 122, 2995–2998;
10.1002/ange.200907162 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 2933–2936;
- 19iR. P. Hughes, J. Fluorine Chem. 2010, 131, 1059–1070;
- 19jJ. Yuan, R. P. Hughes, A. L. Rheingold, Organometallics 2006, 25, 2908–2910.
- 20
- 20aH. Plenio, Chem. Rev. 1997, 97, 3363–3384, and references herein;
- 20bK. Stanek, B. Czarniecki, R. Aardoom, H. Rüegger, A. Togni, Organometallics 2010, 29, 2540–2546.
- 21
- 21aS. Erhardt, S. A. Macgregor, J. Am. Chem. Soc. 2008, 130, 15490–15498;
- 21bA. Nova, S. Erhardt, N. A. Jasim, R. N. Perutz, S. A. Macgregor, J. E. McGrady, A. C. Whitwood, J. Am. Chem. Soc. 2008, 130, 15499–15511;
- 21cJ. Goodman, S. A. Macgregor, Coord. Chem. Rev. 2010, 254, 1295–1306;
- 21dA. Nova, M. Reinhold, R. N. Perutz, S. A. Macgregor, J. E. McGrady, Organometallics 2010, 29, 1824–1831;
- 21eV. V. Grushin, Acc. Chem. Res. 2010, 43, 160–171;
- 21fM. Teltewskoi, J. A. Panetier, S. A. Macgregor, T. Braun, Angew. Chem. 2010, 122, 4039–4043;
10.1002/ange.201001070 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 3947–3951;
- 21gE. Clot, O. Eisenstein, N. Jasim, S. A. Macgregor, J. E. McGrady, R. N. Perutz, Acc. Chem. Res. 2011, 44, 333–348;
- 21hE. Q. Jiao, F. T. Xia, H. Zhu, Comput. Theor. Chem. 2011, 965, 92–100;
- 21iA. L. Raza, J. Panetier, M. Teltewskoi, S. A. Macgregor, T. Braun, Organometallics 2013, 32, 3795–3807.
- 22See the Supporting Information for supplementary crystallographic data of 3. CCDC 941549 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 23
- 23aT. Kashiwagi, N. Yasuoka, T. Ueki, N. Kasai, M. Kakudo, S. Takahashi, N. Nagihara, Bull. Chem. Soc. Jpn. 1968, 41, 296–303;
- 23bC. Bianchini, D. Masi, C. Mealli, A. Meli, M. Sabat, Organometallics 1985, 4, 1014–1019;
- 23cE. Lindner, B. Keppeler, H. A. Mayer, K. Gierling, R. Fawzi, M. Steimann, J. Organomet. Chem. 1996, 526, 175–183;
- 23dM. Doux, N. Mezailles, L. Ricard, P. Le Floch, Organometallics 2003, 22, 4624–4626;
- 23eJ. S. Anderson, V. M. Iluc, G. L. Hillhouse, Inorg. Chem. 2010, 49, 10203–10207.
- 24A.-K. Jungton, P. Kläring, T. Braun, A. Eißler, Z. Anorg. Allg. Chem. 2012, 638, 505–511.
- 25
- 25aB. W. A. Sharp, J. M. Winfield, J. Chem. Soc. 1965, 2278–2279;
- 25bR. C. Poller, Spectrochim. Acta 1966, 22, 935–939;
- 25cA. C. Sau, L. A. Carpino, R. R. Holmes, J. Organomet. Chem. 1980, 197, 181–197.