Haloacylation of the Quintuple-Bonded Group VI Metal Amidinate Dimers and Disproportionation of Acyl Groups to Form Carbynes†
Han-Gung Chen
Department of Chemistry and Frontier Research Center on Fundamental and Applied Sciences of Matters, National Tsing Hua University, Hsinchu 30013 (Taiwan)
Search for more papers by this authorHsiang-Wen Hsueh
Department of Chemistry and Frontier Research Center on Fundamental and Applied Sciences of Matters, National Tsing Hua University, Hsinchu 30013 (Taiwan)
Search for more papers by this authorTing-Shen Kuo
Department of Chemistry, National Taiwan Normal University, Taipei 11677 (Taiwan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Yi-Chou Tsai
Department of Chemistry and Frontier Research Center on Fundamental and Applied Sciences of Matters, National Tsing Hua University, Hsinchu 30013 (Taiwan)
Department of Chemistry and Frontier Research Center on Fundamental and Applied Sciences of Matters, National Tsing Hua University, Hsinchu 30013 (Taiwan)Search for more papers by this authorHan-Gung Chen
Department of Chemistry and Frontier Research Center on Fundamental and Applied Sciences of Matters, National Tsing Hua University, Hsinchu 30013 (Taiwan)
Search for more papers by this authorHsiang-Wen Hsueh
Department of Chemistry and Frontier Research Center on Fundamental and Applied Sciences of Matters, National Tsing Hua University, Hsinchu 30013 (Taiwan)
Search for more papers by this authorTing-Shen Kuo
Department of Chemistry, National Taiwan Normal University, Taipei 11677 (Taiwan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Yi-Chou Tsai
Department of Chemistry and Frontier Research Center on Fundamental and Applied Sciences of Matters, National Tsing Hua University, Hsinchu 30013 (Taiwan)
Department of Chemistry and Frontier Research Center on Fundamental and Applied Sciences of Matters, National Tsing Hua University, Hsinchu 30013 (Taiwan)Search for more papers by this authorWe are grateful to the National Science Council, Taiwan for financial support under Grant NSC 99-2113-M-007-012-MY3. We also thank the Frontier Research Centre on Fundamental and Applied Sciences of Matters for financial support.
Graphical Abstract
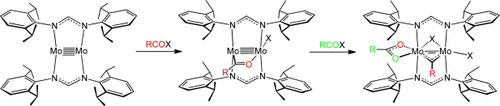
Give me five: Group VI metal quintuple bonds undergo Friedel–Crafts-type haloacylation reactions. Treatment of the quintuple-bonded molybdenum complexes with one equivalent of acyl halides RCOX (R=Me, C6H5, 2-MeC6H4; X=Cl, Br), yields metal–metal quadruple-bonded acyl complexes. Subsequent treatment with a second equivalent of acyl halides causes disproportionation of two acyl groups to give dimolybdenum carbyne carboxylate complexes.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201304750_sm_miscellaneous_information.pdf1.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. E. Pohland, W. R. Benson, Chem. Rev. 1966, 66, 161.
- 2L. J. Gooßen, N. Rodriguez, K. Gooßen, Angew. Chem. 2009, 121, 9770; Angew. Chem. Int. Ed. 2009, 48, 9592.
- 3H. Martens, F. Janssens, G. Hoornaert, Tetrahedron 1975, 31, 177.
- 4F. A. Cotton, D. G. Nocera, Acc. Chem. Res. 2000, 33, 483.
- 5F. A. Cotton, L. A. Murillo, R. A. Walton, Multiple Bond Between Metal Atoms, 3rd ed., Springer, Berlin, 2005.
10.1007/b136230 Google Scholar
- 6T. Nguyen, A. D. Sutton, S. Brynda, J. C. Fettinger, G. J. Long, P. P. Power, Science 2005, 310, 844.
- 7F. R. Wagner, A. Noor, R. Kempe, Nat. Chem. 2009, 1, 529.
- 8Y.-C. Tsai, C.-W. Hsu, J.-S. K. Yu, G.-H. Lee, Y. Wang, T.-S. Kuo, Angew. Chem. 2008, 120, 7360;
10.1002/ange.200801286 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7250.
- 9Y.-L. Huang, D.-Y. Lu, H.-C. Yu, J.-S. K. Yu, C.-W. Hsu, T.-S. Kuo, G.-H. Lee, Y. Wang, Y.-C. Tsai, Angew. Chem. 2012, 124, 7901; Angew. Chem. Int. Ed. 2012, 51, 7781.
- 10E. S. Tamne, A. Noor, S. Qayyum, T. Bauer, R. Kempe, Inorg. Chem. 2013, 52, 329.
- 11C. Schwarzmaier, A. Noor, G. Glatz, M. Zabel, A. Y. Timoshkin, B. M. Cossairt, C. C. Cummins, R. Kempe, M. Scheer, Angew. Chem. 2011, 123, 7421;
10.1002/ange.201102361 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 7283.
- 12C. Ni, B. D. Ellis, G. J. Long, P. P. Power, Chem. Commun. 2009, 2332.
- 13A. Noor, G. Glatz, R. Müller, M. Kaupp, S. Demeshko, R. Kempe, Nat. Chem. 2009, 1, 322.
- 14H.-Z. Chen, S.-C. Liu, C.-H. Yen, J.-S. K. Yu, Y.-J. Shieh, T.-S. Kuo, Y.-C. Tsai, Angew. Chem. 2012, 124, 10488; Angew. Chem. Int. Ed. 2012, 51, 10342.
- 15J. Shen, G. P. A. Yap, J.-P. Werner, K. H. Theopold, Chem. Commun. 2011, 47, 12191.
- 16A. Noor, E. S. Tamne, S. Qayyum, T. Bauer, R. Kempe, Chem. Eur. J. 2011, 17, 6900.
- 17
- 17aM. Carrasco, N. Curado, C. Maya, R. Peloso, A. Rodríguez, E. Ruiz, S. Alvarez, E. Carmona, Angew. Chem. 2013, 125, 3309;
10.1002/ange.201209064 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 3227;
- 17bY.-C. Tsai, H.-Z. Chen, C.-C. Chang, J.-S. K. Yu, G.-H. Lee, Y. Wang, T.-S. Kuo, J. Am. Chem. Soc. 2009, 131, 12534.
- 18C.-W. Hsu, J.-S. K. Yu, G.-H. Lee, Y. Wang, Y.-C. Tsai, Angew. Chem. 2008, 120, 10081; Angew. Chem. Int. Ed. 2008, 47, 9933.
- 19E. Negishi, Acc. Chem. Res. 1987, 20, 65.
- 20Crystallographic data for 4: C57H75Br Mo2N4O: Mr=1104.00, T=200(2) K, monoclinic, space group P21/c, a=14.855(3), b=18.257(3), c=21.137(3) Å, β=109.111(3)°, V=5416.6(15) Å3, Z=4, ρcalcd=1.354 Mg m−3, μ=1.242 mm−1, reflections collected: 34677, independent reflections: 9509 (Rint=0.0460), Final R indices [I>2σ(I)]: R1=0.0375, wR2=0.0879, R indices (all data): R1=0.0613, wR2=0.1021; 5: C58H77ClMo2N4O: Mr=1073.57, T=200(2) K, triclinic, space group P21/c, a=21.489(3), b=21.886(3), c=23.615(3) Å, V=10928(2) Å3, Z=8, ρcalcd=1.305 Mg m−3, μ=0.549 mm−1, reflections collected: 58894, independent reflections: 19334 (Rint=0.0725), Final R indices [I>2σ(I)]: R1=0.0470, wR2=0.0981, R indices (all data): R1=0.0914, wR2=0.1285; 7: C58H77ClCr2N4O Mr=985.69, T=200(2) K, monoclinic, space group P21/c, a=14.6041(6), b=18.1483(7), c=21.8030(9) Å, β=108.847(2)°, V=5468.8(4) Å3, Z=4, ρcalcd=1.197 Mg m−3, μ=0.487 mm−1, reflections collected: 28003, independent reflections: 9598 (Rint=0.0623), Final R indices [I>2σ(I)]: R1=0.0671, wR2=0.1937, R indices (all data): R1=0.1268, wR2=0.2469; 8⋅2.7 C5H12: C77.50H112.40Cl2Mo2N4O2, Mr=1394.89, T=200(2) K, triclinic, space group P
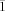 , a=13.103(2), b=14.080(2), c=19.976(3) Å, α=84.099(3), β=80.683(3), γ=81.330(3)°, V=3583.5(10) Å3, Z=2, ρcalcd=1.293 Mg m−3, μ=0.472 mm−1, reflections collected: 20421, independent reflections: 12418 (Rint=0.0295), Final R indices [I>2σ(I)]: R1=0.0583, wR2=0.1697, R indices (all data): R1=0.0822, wR2=0.1826; 9⋅4.1 OEt2: C80.40H121Br2Mo2N4O6.10, Mr=1592.91, T=200(2) K, triclinic, space group P
, a=13.103(2), b=14.080(2), c=19.976(3) Å, α=84.099(3), β=80.683(3), γ=81.330(3)°, V=3583.5(10) Å3, Z=2, ρcalcd=1.293 Mg m−3, μ=0.472 mm−1, reflections collected: 20421, independent reflections: 12418 (Rint=0.0295), Final R indices [I>2σ(I)]: R1=0.0583, wR2=0.1697, R indices (all data): R1=0.0822, wR2=0.1826; 9⋅4.1 OEt2: C80.40H121Br2Mo2N4O6.10, Mr=1592.91, T=200(2) K, triclinic, space group P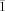 , a=13.0638(3), b=13.8907(3), c=20.4848(4) Å, α=83.4710(10), β=80.8050(10), γ=81.6320(10)°, V=3614.85(13) Å3, Z=2, ρcalcd=1.463 Mg m−3, μ=1.495 mm−1, reflections collected: 31215, independent reflections: 12664 (Rint=0.0340), Final R indices [I>2σ(I)]: R1=0.0408, wR2=0.1106, R indices (all data): R1=0.0599, wR2=0.1198; 11: C54H76Cl2Mo2N4O2, Mr=1075.97, T=200(2) K, triclinic, space group P
, a=13.0638(3), b=13.8907(3), c=20.4848(4) Å, α=83.4710(10), β=80.8050(10), γ=81.6320(10)°, V=3614.85(13) Å3, Z=2, ρcalcd=1.463 Mg m−3, μ=1.495 mm−1, reflections collected: 31215, independent reflections: 12664 (Rint=0.0340), Final R indices [I>2σ(I)]: R1=0.0408, wR2=0.1106, R indices (all data): R1=0.0599, wR2=0.1198; 11: C54H76Cl2Mo2N4O2, Mr=1075.97, T=200(2) K, triclinic, space group P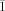 , a=10.6576(16), b=13.8872(13), c=19.530(3) Å, α=84.958(4), β=75.534(6), γ=73.552(5)°, V=2683.9(6) Å3, Z=2, ρcalcd=1.331 Mg⋅m−3, μ=0.609 mm−1, reflections collected: 23358, independent reflections: 9395 (Rint=0.0936), Final R indices [I>2σ(I)]: R1=0.0661, wR2=0.1461, R indices (all data): R1=0.1355, wR2=0.2156; 12⋅2.9 OEt2: C75.60H111Cl2Mo2N4O4.90: Mr=1417.06, T=200(2) K, triclinic, space group P
, a=10.6576(16), b=13.8872(13), c=19.530(3) Å, α=84.958(4), β=75.534(6), γ=73.552(5)°, V=2683.9(6) Å3, Z=2, ρcalcd=1.331 Mg⋅m−3, μ=0.609 mm−1, reflections collected: 23358, independent reflections: 9395 (Rint=0.0936), Final R indices [I>2σ(I)]: R1=0.0661, wR2=0.1461, R indices (all data): R1=0.1355, wR2=0.2156; 12⋅2.9 OEt2: C75.60H111Cl2Mo2N4O4.90: Mr=1417.06, T=200(2) K, triclinic, space group P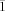 , a=13.0258(19), b=14.299(2), c=19.963(3) Å, α=83.341(2), β=80.195(2), γ=80.632(2)°, V=3600.1(9) Å3, Z=2, ρcalcd=1.307 Mg m−3, μ=0.466 mm−1, reflections collected: 29223, independent reflections: 12601 (Rint=0.0294), Final R indices [I>2σ(I)]: R1=0.0466, wR2=0.1463, R indices (all data): R1=0.0555, wR2=0.1533; 13⋅0.5 OEt2: C67.5H88Cl2Mo2N4O2: Mr=1250.20, T=200(2) K, monoclinic, space group P
, a=13.0258(19), b=14.299(2), c=19.963(3) Å, α=83.341(2), β=80.195(2), γ=80.632(2)°, V=3600.1(9) Å3, Z=2, ρcalcd=1.307 Mg m−3, μ=0.466 mm−1, reflections collected: 29223, independent reflections: 12601 (Rint=0.0294), Final R indices [I>2σ(I)]: R1=0.0466, wR2=0.1463, R indices (all data): R1=0.0555, wR2=0.1533; 13⋅0.5 OEt2: C67.5H88Cl2Mo2N4O2: Mr=1250.20, T=200(2) K, monoclinic, space group P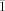 , a=11.9353(12), b=12.8026(12), c=23.213(2) Å, α=83.4280(10), β=84.3730(10), γ=75.2160(10)°, V=3398.3(6) Å3, Z=2, ρcalcd=1.222 Mg m−3, μ=0.490 mm−1, reflections collected: 28012, independent reflections: 11907 (Rint=0.0355), Final R indices [I>2σ(I)]: R1=0.0554, wR2=0.1450, R indices (all data): R1=0.0783, wR2=0.1542; (14+15)⋅4.5 OEt2: C82H125BrClMo2N4O6.50: Mr=1578.10, T=200(2) K, triclinic, space group P
, a=11.9353(12), b=12.8026(12), c=23.213(2) Å, α=83.4280(10), β=84.3730(10), γ=75.2160(10)°, V=3398.3(6) Å3, Z=2, ρcalcd=1.222 Mg m−3, μ=0.490 mm−1, reflections collected: 28012, independent reflections: 11907 (Rint=0.0355), Final R indices [I>2σ(I)]: R1=0.0554, wR2=0.1450, R indices (all data): R1=0.0783, wR2=0.1542; (14+15)⋅4.5 OEt2: C82H125BrClMo2N4O6.50: Mr=1578.10, T=200(2) K, triclinic, space group P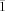 , a=12.920(6), b=13.905(6), c=20.310(10) Å, α=83.858(8), β=80.605(7), γ=82.704(11)°, V=3557(3) Å3, Z=2, ρcalcd=1.474 Mg m−3, μ=1.012 mm−1, reflections collected: 29458, independent reflections: 12546 (Rint=0.0773), Final R indices [I>2σ(I)]: R1=0.0729, wR2=0.2006, R indices (all data): R1=0.1345, wR2=0.2227. CCDC 922848 (4), CCDC 922849 (5), CCDC 929100 (7), CCDC 922846 (9⋅2.7 C5H12), CCDC 922847 (10⋅4.1 OEt2), CCDC 941798 (11), CCDC 922850 (12⋅2.9 OEt2), CCDC 922851 (13⋅0.5 OEt2), CCDC 922852 ((14+15)⋅4.5 OEt2) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via http://www.cam.ac.uk/data_ request/cif.
, a=12.920(6), b=13.905(6), c=20.310(10) Å, α=83.858(8), β=80.605(7), γ=82.704(11)°, V=3557(3) Å3, Z=2, ρcalcd=1.474 Mg m−3, μ=1.012 mm−1, reflections collected: 29458, independent reflections: 12546 (Rint=0.0773), Final R indices [I>2σ(I)]: R1=0.0729, wR2=0.2006, R indices (all data): R1=0.1345, wR2=0.2227. CCDC 922848 (4), CCDC 922849 (5), CCDC 929100 (7), CCDC 922846 (9⋅2.7 C5H12), CCDC 922847 (10⋅4.1 OEt2), CCDC 941798 (11), CCDC 922850 (12⋅2.9 OEt2), CCDC 922851 (13⋅0.5 OEt2), CCDC 922852 ((14+15)⋅4.5 OEt2) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via http://www.cam.ac.uk/data_ request/cif.
- 21F. A. Cotton, L. M. Daniels, E. A. Hillard, C. A. Murillo, Inorg. Chem. 2002, 41, 2466.
- 22J. P. Collman, L. S. Hegedus, J. R. Norton, R. G. Finke, Principles and Applications of Organotransition Metal Chemistry, University Science Books, Mill Valley, 1987.
- 23J. Halpern, Acc. Chem. Res. 1970, 3, 386.
- 24A. Mayr, H. Hoffmeister, Adv. Organomet. Chem. 1991, 32, 227.
- 25D. S. Williams, R. R. Schrock, Organometallics 1994, 13, 2101.
- 26
- 26aM. A. M. Alvarez, E. García, D. García-Vivó, M. E. Martínez, M. Z. Ruiz, Organometallics 2011, 30, 2189;
- 26bM. A. M. Alvarez, E. García, D. García-Vivó, M. E. Martínez, S. Menéndez, M. Z. Ruiz, Organometallics 2010, 29, 710.
- 27M. D. Curtis, Polyhedron 1987, 6, 759.