Total Synthesis and Structural Revision of Viridicatumtoxin B†
Corresponding Author
Prof. Dr. K. C. Nicolaou
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Department of Chemistry and Biochemistry, University of California, San Diego 9500 Gilman Drive, La Jolla, CA 92093 (USA)
Department of Chemistry, BioScience Research Collaborative, Rice University, 6100 Main Street, Houston, TX 77005 (USA)
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)Search for more papers by this authorDr. Christian Nilewski
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Department of Chemistry and Biochemistry, University of California, San Diego 9500 Gilman Drive, La Jolla, CA 92093 (USA)
Department of Chemistry, BioScience Research Collaborative, Rice University, 6100 Main Street, Houston, TX 77005 (USA)
These authors contributed equally to this work.
Search for more papers by this authorChristopher R. H. Hale
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Department of Chemistry and Biochemistry, University of California, San Diego 9500 Gilman Drive, La Jolla, CA 92093 (USA)
Department of Chemistry, BioScience Research Collaborative, Rice University, 6100 Main Street, Houston, TX 77005 (USA)
These authors contributed equally to this work.
Search for more papers by this authorDr. Heraklidia A. Ioannidou
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Department of Chemistry and Biochemistry, University of California, San Diego 9500 Gilman Drive, La Jolla, CA 92093 (USA)
Department of Chemistry, BioScience Research Collaborative, Rice University, 6100 Main Street, Houston, TX 77005 (USA)
Search for more papers by this authorDr. Abdelatif ElMarrouni
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Department of Chemistry and Biochemistry, University of California, San Diego 9500 Gilman Drive, La Jolla, CA 92093 (USA)
Department of Chemistry, BioScience Research Collaborative, Rice University, 6100 Main Street, Houston, TX 77005 (USA)
Search for more papers by this authorLizanne G. Koch
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Department of Chemistry and Biochemistry, University of California, San Diego 9500 Gilman Drive, La Jolla, CA 92093 (USA)
Department of Chemistry, BioScience Research Collaborative, Rice University, 6100 Main Street, Houston, TX 77005 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. K. C. Nicolaou
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Department of Chemistry and Biochemistry, University of California, San Diego 9500 Gilman Drive, La Jolla, CA 92093 (USA)
Department of Chemistry, BioScience Research Collaborative, Rice University, 6100 Main Street, Houston, TX 77005 (USA)
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)Search for more papers by this authorDr. Christian Nilewski
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Department of Chemistry and Biochemistry, University of California, San Diego 9500 Gilman Drive, La Jolla, CA 92093 (USA)
Department of Chemistry, BioScience Research Collaborative, Rice University, 6100 Main Street, Houston, TX 77005 (USA)
These authors contributed equally to this work.
Search for more papers by this authorChristopher R. H. Hale
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Department of Chemistry and Biochemistry, University of California, San Diego 9500 Gilman Drive, La Jolla, CA 92093 (USA)
Department of Chemistry, BioScience Research Collaborative, Rice University, 6100 Main Street, Houston, TX 77005 (USA)
These authors contributed equally to this work.
Search for more papers by this authorDr. Heraklidia A. Ioannidou
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Department of Chemistry and Biochemistry, University of California, San Diego 9500 Gilman Drive, La Jolla, CA 92093 (USA)
Department of Chemistry, BioScience Research Collaborative, Rice University, 6100 Main Street, Houston, TX 77005 (USA)
Search for more papers by this authorDr. Abdelatif ElMarrouni
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Department of Chemistry and Biochemistry, University of California, San Diego 9500 Gilman Drive, La Jolla, CA 92093 (USA)
Department of Chemistry, BioScience Research Collaborative, Rice University, 6100 Main Street, Houston, TX 77005 (USA)
Search for more papers by this authorLizanne G. Koch
Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Department of Chemistry and Biochemistry, University of California, San Diego 9500 Gilman Drive, La Jolla, CA 92093 (USA)
Department of Chemistry, BioScience Research Collaborative, Rice University, 6100 Main Street, Houston, TX 77005 (USA)
Search for more papers by this authorFinancial support was provided by the National Institutes of Health (USA) (grant AI055475-11) and the Skaggs Institute of Research. Fellowships to C.N. (Feodor Lynen Research Fellowship, Alexander von Humboldt Foundation), C.R.H.H. (NSF Graduate Research Fellowship), and A.E. (Fundación Alfonso Martín Escudero) are gratefully appreciated. We thank Dr. R. K. Chadha for X-ray crystallographic assistance and Dr. D. H. Huang and Dr. L. Pasternack for NMR spectroscopic assistance. We thank Prof. W.-G. Kim for graciously providing scanned NMR spectra of natural viridicatumtoxin B.
Graphical Abstract
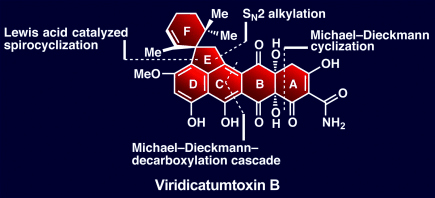
Will the real viridicatumtoxin B please stand up: The total synthesis of viridicatumtoxin B resulted in its structural revision and opens the way for analogue construction and biological evaluation of this complex tetracycline-like antibiotic. The highly convergent strategy employed allows for swift construction of the entire carbocyclic framework of the molecule.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201304691_sm_miscellaneous_information.pdf10 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aF. von Nussbaum, M. Brands, B. Hinzen, S. Weigand, D. Häbich, Angew. Chem. 2006, 118, 5194–5254;
10.1002/ange.200600350 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5072–5129;
- 1bK. C. Nicolaou, J. S. Chen, D. J. Edmonds, A. A. Estrada, Angew. Chem. 2009, 121, 670–732;
10.1002/ange.200801695 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 660–719.
- 2
- 2aC. Walsh, Antibiotics, Actions, Origins, Resistance, 1st ed., ASM, Washington, DC, 2003;
- 2bS. Rachakonda, L. Cartee, Curr. Med. Chem. 2004, 11, 775–793;
- 2cS. B. Levy, B. Marshall, Nat. Med. 2004, 10, S 122–S129;
- 2dC. Nathan, Nature 2004, 431, 899–902.
- 3
- 3aL. H. Conover, K. Butler, J. D. Johnston, J. J. Korst, R. B. Woodward, J. Am. Chem. Soc. 1962, 84, 3222–3224;
- 3bH. Muxfeldt, W. Rogalski, J. Am. Chem. Soc. 1965, 87, 933–934;
- 3cA. I. Gurevich, M. G. Karapetyan, M. N. Kolosov, V. G. Korobko, V. V. Onoprienko, S. A. Popravko, M. M. Shemyakin, Tetrahedron Lett. 1967, 8, 131–134;
10.1016/S0040-4039(00)90501-X Google Scholar
- 3dJ. J. Korst, J. D. Johnston, K. Butler, E. J. Bianco, L. H. Conover, R. B. Woodward, J. Am. Chem. Soc. 1968, 90, 439–457;
- 3eH. Muxfeldt, G. Hardtmann, F. Kathawala, E. Vedejs, J. B. Mooberry, J. Am. Chem. Soc. 1968, 90, 6534–6536;
- 3fD. H. R. Barton, P. D. Magnus, T. Hase, J. Chem. Soc. C 1971, 2215–2225;
- 3gH. Muxfeldt, G. Haas, G. Hardtmann, F. Kathawala, J. B. Mooberry, E. Vedejs, J. Am. Chem. Soc. 1979, 101, 689–701;
- 3hH. H. Wasserman, T.-J. Lu, A. I. Scott, J. Am. Chem. Soc. 1986, 108, 4237–4238;
- 3iG. Stork, J. J. La Clair, P. Spargo, R. P. Nargund, N. Totah, J. Am. Chem. Soc. 1996, 118, 5304–5305;
- 3jK. Tatsuta, T. Yoshimoto, H. Gunji, Y. Okado, M. Takahashi, Chem. Lett. 2000, 646–647;
- 3kJ. S. Wzorek, T. F. Knöpfel, I. Sapountzis, D. A. Evans, Org. Lett. 2012, 14, 5840–5843.
- 4
- 4aM. G. Charest, D. R. Siegel, A. G. Myers, J. Am. Chem. Soc. 2005, 127, 8292–8293;
- 4bM. G. Charest, C. D. Lerner, J. D. Brubaker, D. R. Siegel, A. G. Myers, Science 2005, 308, 395–398;
- 4cJ. D. Brubaker, A. G. Myers, Org. Lett. 2007, 9, 3523–3525;
- 4dC. Sun, Q. Wang, J. D. Brubaker, P. M. Wright, C. D. Lerner, K. Noson, M. Charest, D. R. Siegel, Y.-M. Wang, A. G. Myers, J. Am. Chem. Soc. 2008, 130, 17913–17927;
- 4eP. M. Wright, A. G. Myers, Tetrahedron 2011, 67, 9853–9869;
- 4fD. A. Kummer, D. Li, A. Dion, A. G. Myers, Chem. Sci. 2011, 2, 1710–1718.
- 5
- 5aR. D. Hutchison, P. S. Steyn, S. J. Van Rensburg, Toxicol. Appl. Pharmacol. 1973, 24, 507–509. We propose that viridicatumtoxin be renamed “viridicatumtoxin A.” It has been suggested that viridicatumtoxin A was isolated from Penicillium aethiopicum and not the originally reported Penicillium viridicatum; see
- 5bJ. C. Frisvad, O. Filtenborg, Mycologia 1989, 81, 837–861.
- 6
- 6aC. Kabuto, J. V. Silverton, T. Akiyama, U. Sankawa, R. D. Hutchison, P. S. Steyn, R. Vleggaar, J. Chem. Soc. Chem. Commun. 1976, 728–729;
- 6bJ. V. Silverton, C. Kabuto, T. Akiyama, Acta Crystallogr. Sect. B 1982, 38, 3032–3037.
- 7C.-J. Zheng, H.-E. Yu, E.-H. Kim, W.-G. Kim, J. Antibiot. 2008, 61, 633–637.
- 8J. Inokoshi, Y. Nakamura, Z. Hongbin, R. Uchida, K. Nonaka, R. Masuma, H. Tomoda, J. Antibiot. 2013, 66, 37–41.
- 9N. Koyama, J. Inokoshi, H. Tomoda, Molecules 2013, 18, 204–224.
- 10
- 10aA. E. de Jesus, W. E. Hull, P. S. Steyn, F. R. van Heerden, R. Vleggaar, J. Chem. Soc. Chem. Commun. 1982, 902–904;
- 10bR. M. Horak, V. J. Maharaj, S. F. Marais, F. R. van Heerden, R. Vleggaar, J. Chem. Soc. Chem. Commun. 1988, 1562–1564;
- 10cH. Zhou, Y. Li, Y. Tang, Nat. Prod. Rep. 2010, 27, 839–868;
- 10dY.-H. Chooi, R. Cacho, Y. Tang, Chem. Biol. 2010, 17, 483–494;
- 10eY. Li, Y.-H. Chooi, Y. Sheng, J. S. Valentine, Y. Tang, J. Am. Chem. Soc. 2011, 133, 15773–15785;
- 10fY.-H. Chooi, P. Wang, J. Fang, Y. Li, K. Wu, P. Wang, Y. Tang, J. Am. Chem. Soc. 2012, 134, 9428–9437.
- 11Prepared in one step from 4-methoxyphenol: A. Pelter, S. Elgendy, Tetrahedron Lett. 1988, 29, 677–680.
- 12I. Kadás, G. Árvai, G. Horváth, Org. Prep. Proced. Int. 1998, 30, 79–85.
- 13W. E. Bauta, D. P. Lovett, W. R. Cantrell, Jr., B. D. Burke, J. Org. Chem. 2003, 68, 5967–5973.
- 14G. Stork, A. A. Hagedorn III, J. Am. Chem. Soc. 1978, 100, 3609–3611.
- 15M. W. Rathke, P. J. Cowan, J. Org. Chem. 1985, 50, 2622–2624.
- 16V. P. Fitzjarrald, R. Pongdee, Tetrahedron Lett. 2007, 48, 3553–3557.
- 17Mechanistic studies of the one-pot procedure involving the use of DBU on related systems suggested a stepwise pathway. The use of a strong base (i.e. NaH or LDA) with homophthalic anhydrides and quinones (Tamura Diels–Alder reaction) is typically depicted as a concerted [4+2] cycloaddition, but the synchronicity of the reaction has been questioned:
- 17aC. D. Cox, T. Siu, S. J. Danishefsky, Angew. Chem. 2003, 115, 5783–5787;
10.1002/ange.200352591 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 5625–5629;
- 17bM. González-López, J. T. Shaw, Chem. Rev. 2009, 109, 164–189.
- 18CCDC 941202, 941203, and 945867 contain the supplementary crystallographic data for compounds 23, 29, and 38, respectively for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Compounds 23 and 29 were prepared by similar routes to those described herein and will be reported in the full account of this work.
- 19
- 19aJ. D. White, E. G. Nolen, Jr., C. H. Miller, J. Org. Chem. 1986, 51, 1150–1152;
- 19bJ. D. White, F. W. J. Demnitz, Q. Xu, W. H. C. Martin, Org. Lett. 2008, 10, 2833–2836.
- 20aC. Gioeli, N. Balgobin, S. Josephson, J. B. Chattopadhyaya, Tetrahedron Lett. 1981, 22, 969–972;
- 20bK. C. Nicolaou, K. R. Reddy, G. Skokotas, F. Sato, X. Y. Xiao, C. K. Hwang, J. Am. Chem. Soc. 1993, 115, 3558–3575;
- 20cS. E. Denmark, T. Kobayashi, C. S. Regens, Tetrahedron 2010, 66, 4745–4759.
- 21A. Fürstner, H. Weintritt, J. Am. Chem. Soc. 1998, 120, 2817–2825.
- 22W. Adam, A. K. Smerz, Tetrahedron 1996, 52, 5799–5804.
- 23M. Gibert, M. Ferrer, F. Sánchez-Baeza, A. Messeguer, Tetrahedron 1997, 53, 8643–8650.
- 24Electron-rich α-methoxystyrenes are known to be very labile, even at near-neutral pH values; see, for example: J. P. Richard, K. B. Williams, J. Am. Chem. Soc. 2007, 129, 6952–6961.
- 25A. I. Scott, E. Yamaguchi, S.-K. Chung, Tetrahedron Lett. 1975, 16, 1369–1372.
10.1016/S0040-4039(00)72145-9 Google Scholar
- 26D. A. Evans, K. T. Chapman, E. M. Carreira, J. Am. Chem. Soc. 1988, 110, 3560–3578.
- 27K. Tatsuta, T. Fukuda, T. Ishimori, R. Yachi, S. Yoshida, H. Hashimoto, S. Hosokawa, Tetrahedron Lett. 2012, 53, 422–425.
- 28F. A. Davis, O. D. Stringer, J. Org. Chem. 1982, 47, 1774–1775.
- 29D. B. Dess, J. C. Martin, J. Org. Chem. 1983, 48, 4155–4156.
- 30We thank Professor W.-G. Kim for kindly providing us with the 1H and 13C NMR spectra of natural viridicatumtoxin B obtained at 300 MHz and 226 MHz, respectively, from a small sample of natural material. These spectroscopic data were in accord with those obtained by us at 600 MHz and 151 MHz for synthetic material, thus confirming the identity of the two samples (except for the racemic nature of ours). Furthermore, we noted certain discrepancies in the reported chemical shift values in Ref. [7] compared to those actually found in the provided spectra of the natural product. In particular, the originally unreported carbonyl resonance near δ=194 ppm which we assigned as C5 was indeed present in the scanned authentic spectrum of the natural product. For the spectra of the natural and synthetic materials and further details, see the Supporting Information.