Total Synthesis of Vinigrol†
Qingliang Yang
Department of Chemistry and Chemical Biology, Cornell University, Baker Laboratory, Ithaca, NY 14853-1301 (USA)
Search for more papers by this authorCorresponding Author
Prof. Jon T. Njardarson
Department of Chemistry and Biochemistry, University of Arizona, 1306 E. University Blvd., Tucson, AZ 85721 (USA)
Department of Chemistry and Biochemistry, University of Arizona, 1306 E. University Blvd., Tucson, AZ 85721 (USA)Search for more papers by this authorDr. Cristian Draghici
Department of Chemistry and Chemical Biology, Cornell University, Baker Laboratory, Ithaca, NY 14853-1301 (USA)
Search for more papers by this authorDr. Fang Li
Department of Chemistry and Biochemistry, University of Arizona, 1306 E. University Blvd., Tucson, AZ 85721 (USA)
Search for more papers by this authorQingliang Yang
Department of Chemistry and Chemical Biology, Cornell University, Baker Laboratory, Ithaca, NY 14853-1301 (USA)
Search for more papers by this authorCorresponding Author
Prof. Jon T. Njardarson
Department of Chemistry and Biochemistry, University of Arizona, 1306 E. University Blvd., Tucson, AZ 85721 (USA)
Department of Chemistry and Biochemistry, University of Arizona, 1306 E. University Blvd., Tucson, AZ 85721 (USA)Search for more papers by this authorDr. Cristian Draghici
Department of Chemistry and Chemical Biology, Cornell University, Baker Laboratory, Ithaca, NY 14853-1301 (USA)
Search for more papers by this authorDr. Fang Li
Department of Chemistry and Biochemistry, University of Arizona, 1306 E. University Blvd., Tucson, AZ 85721 (USA)
Search for more papers by this authorWe thank the NIH-NIGMS (RO1 GM086584) for generous financial support of this program.
Graphical Abstract
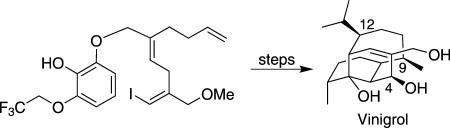
Carbocyclic cage fight: The substrate-controlled total synthesis of vinigrol features a strategic oxidative dearomatization/Diels–Alder cycloaddition reaction and a subsequent palladium-catalyzed cyclization cascade to construct the carbocyclic core. The C4, C9, and C12 stereocenters were installed using either reduction or oxidation reactions, and the diterpenoid core was unraveled by a ring fragmentation reaction.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201304624_sm_miscellaneous_information.pdf3.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1I. Uchida, T. Ando, N. Fukami, K. Yoshida, M. Hashimoto, T. Tada, S. Koda, Y. Morimoto, J. Org. Chem. 1987, 52, 5292–5293.
- 2
- 2aT. Ando, Y. Tsurumi, N. Ohata, I. Uchida, K. Yoshida, M. Okahura, J. Antibiot. 1988, 41, 25–30;
- 2bT. Ando, K. Yoshida, M. Okahura, J. Antibiot. 1988, 41, 31–35.
- 3D. B. Norris, P. Depledge, A. P. Jackson, PCT Int. Appl. WO 9107 953, 1991.
- 4
- 4aH. Nakajima, N. Yamamoto, T. Kaizu, T. Kino, Jpn. Kokai Tokkyo Koho JP 07206668, 1995;
- 4bJ. T. Keane, PCT Int. Appl. WO 20011000229, 2001;
- 4cH. Onodera, M. Ichimura, K. Sakurada, A. Kawabata, T. Ota, PCT Int. Appl. WO 2006077954, 2006;
- 4dH. Onodera, M. Ichimura, K. Sakurada, A. Kawabata, T. Ota, PCT Int. Appl. WO 2006077954.
- 5
- 5aJ.-F. Devaux, I. Hanna, J.-Y. Lallemand, J. Org. Chem. 1993, 58, 2349–2350;
- 5bJ.-F. Devaux, I. Hanna, P. Fraisse, J.-Y. Lallemand, Tetrahedron Lett. 1995, 36, 9471–9474;
- 5cJ.-F. Devaux, I. Hanna, J.-Y. Lallemand, T. Prange, J. Chem. Res. Synth. 1996, 32–33;
- 5dG. Mehta, K. S. Reddy, Synlett 1996, 625–627;
- 5eM. Kito, T. Sakai, N. Haruta, H. Shirahama, F. Matsuda, Synlett 1996, 1057–1060;
- 5fJ.-F. Devaux, I. Hanna, J.-Y. Lallemand, J. Org. Chem. 1997, 62, 5062–5068;
- 5gM. Kito, T. Sakai, H. Shirahama, M. Miyashita, F. Matsuda, Synlett 1997, 219–220;
- 5hF. Matsuda, T. Sakai, N. Okada, M. Miyashita, Tetrahedron Lett. 1998, 39, 863–864;
- 5iF. Matsuda, M. Kito, T. Sakai, N. Okada, M. Miyashita, H. Shirahama, Tetrahedron 1999, 55, 14369–14380;
- 5jL. Gentric, I. Hanna, L. Ricard, Org. Lett. 2003, 5, 1139–1142;
- 5kL. Gentric, I. Hanna, A. Huboux, R. Zaghdoudi, Org. Lett. 2003, 5, 3631–3634;
- 5lL. A. Paquette, R. Guevel, S. Sakamoto, I. H. Kim, J. Crawford, J. Org. Chem. 2003, 68, 6096–6107;
- 5mL. Morency, L. Barriault, Tetrahedron Lett. 2004, 45, 6105–6107;
- 5nL. A. Paquette, I. Efremov, Z. Liu, J. Org. Chem. 2005, 70, 505–509;
- 5oL. A. Paquette, I. Efremov, J. Org. Chem. 2005, 70, 510–513;
- 5pL. A. Paquette, Z. Liu, I. Efremov, J. Org. Chem. 2005, 70, 514–518;
- 5qL. Morency, L. Barriault, J. Org. Chem. 2005, 70, 8841–8853;
- 5rC. M. Grisé, G. Tessier, L. Barriault, Org. Lett. 2007, 9, 1545–1548;
- 5sM. S. Souweha, G. D. Enright, A. G. Fallis, Org. Lett. 2007, 9, 5163–5166;
- 5tT. J. Maimone, A.-F. Voica, P. S. Baran, Angew. Chem. 2008, 120, 3097–3099;
10.1002/ange.200800167 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3054–3056; for reviews on vinigrol approaches, consult:
- 5uG. Tessier, L. Barriault, Org. Prep. Proced. Int. 2007, 37, 313–353;
- 5vM. Harmata, N. L. Calkins, Chemtracts 2009, 22, 205–209;
- 5wJ.-Y. Lu, D. G. Hall, Angew. Chem. 2010, 122, 2336–2338; Angew. Chem. Int. Ed. 2010, 49, 2286–2288;
- 5xA. D. Huters, N. K. Garg, Chem. Eur. J. 2010, 16, 8586–8595.
- 6T. J. Maimone, J. Shi, S. Ashida, P. S. Baran, J. Am. Chem. Soc. 2009, 131, 17066–17067.
- 7J. Poulin, C. M. Grise-Bard, L. Barriault, Angew. Chem. 2012, 124, 2153–2156;
10.1002/ange.201108779 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 2111–2114.
- 8
- 8aJ. G. M. Morton, L. D. Kwon, J. D. Freeman, J. T. Njardarson, Synlett 2009, 23–27;
- 8bJ. G. M. Morton, L. D. Kwon, J. D. Freeman, J. T. Njardarson, Tetrahedron Lett. 2009, 50, 1684–1686;
- 8cJ. G. M. Morton, C. Draghici, J. T. Njardarson, Org. Lett. 2009, 11, 4492–4495.
- 9A. Hadfield, H. Schweitzer, M. P. Trova, K. Green, Synth. Commun. 1994, 24, 1025–1028.
- 10The compound 8 can be accessed in two steps from commercially available 3-(trimethylsilyl)propargyl alcohol:
- 10aL. Labaudiniere, J. Hanaizi, J.-F. Normant, J. Org. Chem. 1992, 57, 6903–6908;
- 10bJ. G. Duboudin, B. Jousseaume, J. Organomet. Chem. 1979, 168, 1–11.
- 11R. C. Petter, S. Banerjee, S. Englard, J. Org. Chem. 1990, 55, 3088–3097.
- 12Using boric acid as an additive was critical to the success of the Dakin oxidation: A. Roy, K. R. Reddy, P. K. Mohanta, H. Ila, H. Junjappa, Synth. Commun. 1999, 29, 3781–3791.
- 13The outcome of this Grignard addition was very sensitive to solvents. For example when ether was replaced with THF or MgBr2 was omitted the diastereoselectivity plummeted.
- 14B. Wüstenberg, A. Pfaltz, Adv. Synth. Catal. 2008, 350, 174–178. We are grateful to Professor Andreas Pfaltz for sending us samples of his iridium catalysts.
- 15D. A. Evans, K. T. Chapman, Tetrahedron Lett. 1986, 27, 5939–5942.
- 16P. S. Wharton, J. Org. Chem. 1961, 26, 4781–4782.
- 17M. Imamoto, Y. Sugiura, N. Takiyama, Tetrahedron Lett. 1984, 25, 4233–4236.
- 18G. M. Atkins, E. M. Burgess, J. Am. Chem. Soc. 1968, 90, 4744–4745.
- 19A. L. Wilds, J. A. Johnson, R. E. Sutton, J. Am. Chem. Soc. 1950, 72, 5524–5529.
- 20
- 20aP. C. Bulman, T. J. McCarthy, Comp. Org. Synth. 1991, 7, 83;
- 20bS. Hanessian, J. Szychowski, J. P. Maianti, Org. Lett. 2009, 11, 429–432.
- 21L. A. Sarandeses, A. Mourino, J.-L. Luche, J. Chem. Soc. Chem. Commun. 1991, 818–820.
- 22
- 22aT. Nakai, K. Tanaka, N. Ishikawa, Chem. Lett. 1976, 1263–1266;
- 22bK. Tanaka, S. Shiraishi, T. Nakai, N. Ishikawa, Tetrahedron Lett. 1978, 19, 3103–3106.
10.1016/S0040-4039(01)94954-8 Google Scholar
- 23W. A. Herrmann, S. J. Eder, W. Scherer, Angew. Chem. 1992, 104, 1371–1373; Angew. Chem. Int. Ed. Engl. 1992, 31, 1345–1347.
- 24Our spectral and physical data excellently matched that of a sample of vinigrol graciously shared with us by Professor Phil S. Baran (see the Supporting Information for more details).