Catalytic, Enantioselective, and Highly Chemoselective Bromocyclization of Olefinic Dicarbonyl Compounds†
Yi Zhao
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore) http://www.chemistry.nus.edu.sg/people/academic_staff/yeungyy.htm
Search for more papers by this authorDr. Xiaojian Jiang
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore) http://www.chemistry.nus.edu.sg/people/academic_staff/yeungyy.htm
Search for more papers by this authorCorresponding Author
Prof. Dr. Ying-Yeung Yeung
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore) http://www.chemistry.nus.edu.sg/people/academic_staff/yeungyy.htm
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore) http://www.chemistry.nus.edu.sg/people/academic_staff/yeungyy.htmSearch for more papers by this authorYi Zhao
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore) http://www.chemistry.nus.edu.sg/people/academic_staff/yeungyy.htm
Search for more papers by this authorDr. Xiaojian Jiang
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore) http://www.chemistry.nus.edu.sg/people/academic_staff/yeungyy.htm
Search for more papers by this authorCorresponding Author
Prof. Dr. Ying-Yeung Yeung
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore) http://www.chemistry.nus.edu.sg/people/academic_staff/yeungyy.htm
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore) http://www.chemistry.nus.edu.sg/people/academic_staff/yeungyy.htmSearch for more papers by this authorWe thank the National University of Singapore (grant no. 143-000-509-112), ASTAR-Public Sector Funding (grant no. 143-000-536-305), and GSK-EDB for financial support. We are also grateful for scholarships to Y. Zhao and X. Jiang (NUS Research Scholarship). Special thanks goes to Dr. J. Chen (NUS) for her assistance on the mechanistic investigation and Dr. Ji’En Wu (CMMAC, NUS) for his help on the NMR analysis.
Graphical Abstract
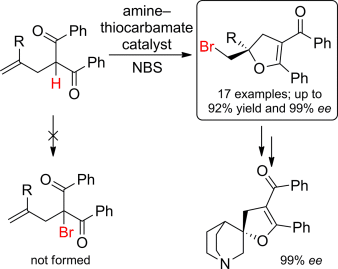
Overriding preferences: An amine–thiocarbamate catalyst can mediate the facile, efficient, and highly enantioselective bromocyclization of olefinic 1,3-dicarbonyl compounds. In the presence of the bifunctional catalyst, the bromination occurs chemoselectively at the olefinic moiety rather than at the carbon atom in the α-position to the carbonyl units.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201304107_sm_miscellaneous_information.pdf3.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aS. E. Denmark, W. E. Kuester, M. T. Burk, Angew. Chem. 2012, 124, 11098–11113;
10.1002/ange.201204347 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 10938–10953;
- 1bG. Li, S. R. S. Saibabu Kotti, C. Timmons, Eur. J. Org. Chem. 2007, 2745–2748;
- 1cS. Ranganathan, K. M. Muraleedharan, N. K. Vaish, N. Jayaraman, Tetrahedron 2004, 60, 5273–5308;
- 1dA. N. French, S. Bissmire, T. Wirth, Chem. Soc. Rev. 2004, 33, 354–362;
- 1eG. F. Chen, S. M. Ma, Angew. Chem. 2010, 122, 8484–8486; Angew. Chem. Int. Ed. 2010, 49, 8306–8308;
- 1fF. Rodríguez, F. J. Fañanás in Handbook of Cyclization Reactions, Vol. 4 (Ed.: ), Wiley-VCH, New York, 2010, pp. 951–990;
- 1gA. Castellanos, S. P. Fletcher, Chem. Eur. J. 2011, 17, 5766–5776;
- 1hC. K. Tan, L. Zhou, Y.-Y. Yeung, Synlett 2011, 1335–1339;
- 1iU. Hennecke, Chem. Asian J. 2012, 7, 456–465; for some selected early efforts, see:
- 1jD. C. Whitehead, R. Yousefi, A. Jaganathan, B. Borhan, J. Am. Chem. Soc. 2010, 132, 3298–3300;
- 1kW. Zhang, S. Zheng, N. Liu, J. B. Werness, I. A. Guzei, W. Tang, J. Am. Chem. Soc. 2010, 132, 3664–3665;
- 1lG. E. Veitch, E. N. Jacobsen, Angew. Chem. 2010, 122, 7490–7493;
10.1002/ange.201003681 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7332–7335;
- 1mK. Murai, T. Matsushita, A. Nakamura, S. Fukushima, M. Shimura, H. Fujioka, Angew. Chem. 2010, 122, 9360–9363;
10.1002/ange.201005409 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 9174–9177; for some recent examples, see:
- 1nY. Cai, X. Liu, Y. Hui, J. Jiang, W. Wang, W. Chen, L. Lin, X. Feng, Angew. Chem. 2010, 122, 6296–6300; Angew. Chem. Int. Ed. 2010, 49, 6160–6164;
- 1oY. Cai, X. Liu, J. Jiang, W. Chen, L. Lin, X. Feng, J. Am. Chem. Soc. 2011, 133, 5636–5639;
- 1pD. Huang, H. Wang, F. Xue, H. Guan, L. Li, X. Peng, Y. Shi, Org. Lett. 2011, 13, 6350–6353;
- 1qU. Hennecke, C. H. Müller, R. Fröhlich, Org. Lett. 2011, 13, 860–863;
- 1rC. Fang, D. H. Paull, J. C. Hethcox, C. R. Shugrue, S. F. Martin, Org. Lett. 2012, 14, 6290–6293;
- 1sJ. E. Tungen, J. M. J. Nolsøe, T. V. Hansen, Org. Lett. 2012, 14, 5884–5887;
- 1tM. C. Dobish, J. N. Johnston, J. Am. Chem. Soc. 2012, 134, 6068–6071;
- 1uK. Murai, A. Nakamura, T. Matsushita, M. Shimura, H. Fujioka, Chem. Eur. J. 2012, 18, 8448–8453;
- 1vW. Zhang, N. Liu, C. M. Schienebeck, K. Decloux, S. Zheng, J. B. Werness, W. Tang, Chem. Eur. J. 2012, 18, 7296–7305;
- 1wD. H. Paull, C. Fang, J. R. Donald, A. D. Pansick, S. F. Martin, J. Am. Chem. Soc. 2012, 134, 11128–11131;
- 1xK. Ikeuchi, S. Ido, S. Yoshimura, T. Asakawa, M. Inai, Y. Hamashima, T. Kan, Org. Lett. 2012, 14, 6016–6019;
- 1yS. E. Denmark, M. T. Burk, Org. Lett. 2012, 14, 256–259;
- 1zY.-M. Wang, J. Wu, C. Hoong, V. Rauniyar, F. D. Toste, J. Am. Chem. Soc. 2012, 134, 12928–12931.
- 2
- 2aG. W. Gordon, J. Nat. Prod. 1992, 55, 1353–1395;
- 2bG. W. Gordon, Chem. Soc. Rev. 1999, 28, 335–346;
- 2cG. W. Gordon, Acc. Chem. Res. 1999, 31, 141–152;
- 2dG. W. Gordon, J. Chem. Educ. 2004, 81, 1441–1449;
- 2eB.-G. Wang, J. B. Gloer, N.-Y. Ji, J.-C. Zhao, Chem. Rev. 2013, 113, 3632–3685.
- 3
- 3aM. Santelli, C. Ollivier in Comprehensive Organic Functional Group Transformations II, Vol. 1 (Eds.: ), Elsevier, Oxford, 2005, p. 121;
10.1016/B0-08-044655-8/00004-0 Google Scholar
- 3b Advances in Organobromine Chemistry I, Vol. 1 (Eds.: ), Elsevier, Oxford, 1991.
- 4For examples of other kinds of substrates, see:
- 4aA. Sakakura, A. Ukai, K. Ishihara, Nature 2007, 445, 900–903;
- 4bK. C. Nicolaou, N. L. Simmons, Y. Ying, P. M. Heretsch, J. S. Chen, J. Am. Chem. Soc. 2011, 133, 8134–8137;
- 4cZ.-M. Chen, Q.-W. Zhang, Z.-H. Chen, H. Li, Y.-Q. Tu, F.-M. Zhang, J.-M. Tian, J. Am. Chem. Soc. 2011, 133, 8818–8821;
- 4dH. Li, F.-M. Zhang, Y.-Q. Tu, Q.-W. Zhang, Z.-M. Chen, Z.-H. Chen, J. Li, Chem. Sci. 2011, 2, 1839–1841;
- 4eO. Lozano, G. Blessley, T. Martinez del Campo, A. L. Thompson, G. T. Giuffredi, M. Bettati, M. Walker, R. Borman, V. Gouverneur, Angew. Chem. 2011, 123, 8255–8259; Angew. Chem. Int. Ed. 2011, 50, 8105–8109;
- 4fC. H. Müller, M. Wilking, A. Rühlmann, B. Wibbeling, U. Hennecke, Synlett 2011, 2043–2047;
- 4gA. Alix, C. Lalli, P. Retailleau, G. Masson, J. Am. Chem. Soc. 2012, 134, 10389–10392;
- 4hK. Mori, Y. Ichikawa, M. Kobayashi, Y. Shibata, M. Yamanaka, T. Akiyama, J. Am. Chem. Soc. 2013, 135, 3964–3970.
- 5
- 5aR. Antonioletti, F. Bonadies, A. Scettri, Tetrahedron Lett. 1988, 29, 4987–4990;
- 5bR. Antonioletti, S. Malancona, P. Bovicelli, Tetrahedron 2002, 58, 8825–8831;
- 5cR. Antonioletti, S. Malancona, F. Cattaruzza, P. Bovicelli, Tetrahedron 2003, 59, 1673–1678.
- 6For enantioselective examples, see:
- 6aL. Hintermann, A. Togni, Helv. Chim. Acta 2000, 83, 2425–2435;
- 6bY. Zhang, K. Shibatomi, H. Yamamoto, J. Am. Chem. Soc. 2004, 126, 15038–15039;
- 6cG. Bartoli, M. Bosco, A. Carlone, M. Locatelli, P. Melchiorre, L. Sambri, Angew. Chem. 2005, 117, 6375–6378;
10.1002/ange.200502134 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 6219–6222;
- 6dY. Cai, W. Wang, K. Shen, J. Wang, X. Hu, L. Lin, X. Liu, X. Feng, Chem. Commun. 2010, 46, 1250–1252;
- 6eW. Zheng, Z. Zhang, M. J. Kaplan, J. C. Antilla, J. Am. Chem. Soc. 2011, 133, 3339–3341;
- 6fD. Wang, J.-J. Jiang, R. Zhang, M. Shi, Tetrahedron: Asymmetry 2011, 22, 1133–1141;
- 6gK. Shibatomi, A. Narayama, Y. Soga, T. Muto, S. Iwasa, Org. Lett. 2011, 13, 2944–2947;
- 6hY. K. Kang, H. H. Kim, K. O. Koh, D. Y. Kim, Tetrahedron Lett. 2012, 53, 3811–3814;
- 6iA. Bertogg, L. Hintermann, D. P. Huber, M. Perseghini, M. Sanna, A. Togni, Helv. Chim. Acta 2012, 95, 353–403;
- 6jK. Shibatomi, Y. Soga, A. Narayama, I. Fujisawa, S. Iwasa, J. Am. Chem. Soc. 2012, 134, 9836–9839;
- 6kJ. Li, W. Pan, Z. Wang, X. Zhang, K. Ding, Adv. Synth. Catal. 2012, 354, 1980–1986;
- 6lE.-M. Tanzer, W. B. Schweizer, M.-O. Ebert, R. Gilmour, Chem. Eur. J. 2012, 18, 2006–2013; for some non-enantioselective examples, see:
- 6mS. K. Guha, B. Wu, B. S. Kim, W. Baik, S. Koo, Tetrahedron Lett. 2006, 47, 291–293;
- 6nG. H. Hakimelahi, G. Just, Tetrahedron Lett. 1979, 38, 3643–3644;
10.1016/S0040-4039(01)95485-1 Google Scholar
- 6oG. S. Coumbarides, M. Dingjan, J. Eames, N. Weerasooriya, Bull. Chem. Soc. Jpn. 2001, 74, 179–180;
- 6pD. Yang, Y.-L. Yan, B. Lui, J. Org. Chem. 2002, 67, 7429–7431.
- 7We attempted to use 2 a as the source of the bromine atom in the formation of 3 a, but the reaction did not occur. For details, see Scheme 4.
- 8DABCO and some other amine bases were used as catalysts in the bromocyclization of olefinic acids, which were obtained with excellent diastereoselectivity. For an elegant example, see: W. Zhang, H. Xu, H. Xu, W. Tang, J. Am. Chem. Soc. 2009, 131, 3832–3833.
- 9The structures of these catalysts are given in the Supporting Information.
- 10We have also examined the substrate with a 1,3-di-tert-butylpropane-1,3-dione substituent instead of a 1,3-diphenylpropane-1,3-dione. For details, see the Supporting Information.
- 11
- 11aL. Zhou, C. K. Tan, X. Jiang, F. Chen, Y.-Y. Yeung, J. Am. Chem. Soc. 2010, 132, 15474–15476;
- 11bC. K. Tan, L. Zhou, Y.-Y. Yeung, Org. Lett. 2011, 13, 2738–2741;
- 11cC. K. Tan, F. Chen, Y.-Y. Yeung, Tetrahedron Lett. 2011, 52, 4892–4895;
- 11dL. Zhou, J. Chen, C. K. Tan, Y.-Y. Yeung, J. Am. Chem. Soc. 2011, 133, 9164–9167;
- 11eJ. Chen, L. Zhou, C. K. Tan, Y.-Y. Yeung, J. Org. Chem. 2012, 77, 999–1009;
- 11fJ. Chen, L. Zhou, Y.-Y. Yeung, Org. Biomol. Chem. 2012, 10, 3808–3811;
- 11gC. K. Tan, C. Le, Y.-Y. Yeung, Chem. Commun. 2012, 48, 5793–5795;
- 11hX. Jiang, C. K. Tan, L. Zhou, Y.-Y. Yeung, Angew. Chem. 2012, 124, 7891–7895; Angew. Chem. Int. Ed. 2012, 51, 7771–7775;
- 11iL. Zhou, D. W. Tay, J. Chen, G. Y. C. Leung, Y.-Y. Yeung, Chem. Commun. 2013, 49, 4412–4414;
- 11jF. Chen, C. K. Tan, Y.-Y. Yeung, J. Am. Chem. Soc. 2013, 135, 1232–1235.
- 12CCDC 935968 (3 a) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 13
- 13aC. E. Song, Cinchona Alkaloids in Synthesis & Catalysis, Wiley-VCH, Weinheim, 2009;
10.1002/9783527628179 Google Scholar
- 13bH. Li, Y. Chen, L. Deng in Privileged Chiral Ligands and Catalysts (Ed.: ), Wiley-VCH, Weinheim, 2011, pp. 361–408.
10.1002/9783527635207.ch10 Google Scholar
- 14The bifunctional system appears to be responsible for the high reaction efficiency, as indicated by the results shown in Table 1, entries 6 and 7 (higher efficiency was observed when the amine–thiocarbamate catalyst was used).