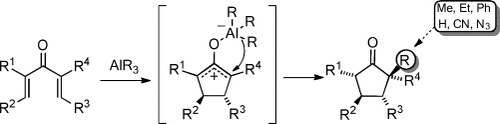
Organoaluminum-Mediated Interrupted Nazarov Reaction†
Yonghoon Kwon
Department of Chemistry, University of Alberta, E3-43 Gunning-Lemieux Chemistry Centre, Edmonton, Alberta, T6G 2G2 (Canada)
Search for more papers by this authorDr. Robert McDonald
X-ray Crystallographic Lab, Department of Chemistry, University of Alberta (Canada)
Search for more papers by this authorCorresponding Author
Prof. Frederick G. West
Department of Chemistry, University of Alberta, E3-43 Gunning-Lemieux Chemistry Centre, Edmonton, Alberta, T6G 2G2 (Canada)
Department of Chemistry, University of Alberta, E3-43 Gunning-Lemieux Chemistry Centre, Edmonton, Alberta, T6G 2G2 (Canada)Search for more papers by this authorYonghoon Kwon
Department of Chemistry, University of Alberta, E3-43 Gunning-Lemieux Chemistry Centre, Edmonton, Alberta, T6G 2G2 (Canada)
Search for more papers by this authorDr. Robert McDonald
X-ray Crystallographic Lab, Department of Chemistry, University of Alberta (Canada)
Search for more papers by this authorCorresponding Author
Prof. Frederick G. West
Department of Chemistry, University of Alberta, E3-43 Gunning-Lemieux Chemistry Centre, Edmonton, Alberta, T6G 2G2 (Canada)
Department of Chemistry, University of Alberta, E3-43 Gunning-Lemieux Chemistry Centre, Edmonton, Alberta, T6G 2G2 (Canada)Search for more papers by this authorWe thank NSERC for support of this work.
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303996_sm_miscellaneous_information.pdf6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Review: W. Nakanishi, F. G. West, Curr. Opin. Drug Discov. Devel. 2009, 12, 732–751.
- 2Recent Reviews:
- 2aT. Vaidya, R. Eisenberg, A. J. Frontier, ChemCatChem 2011, 3, 1531–1548;
- 2bN. Shimada, C. Stewart, M. A. Tius, Tetrahedron 2011, 67, 5851–5870.
- 3Recent examples:
- 3aT. N. Grant, F. G. West, J. Am. Chem. Soc. 2006, 128, 9348–9349;
- 3bT. Jin, Y. Yamamoto, Org. Lett. 2008, 10, 3137–3139;
- 3cY.-K. Wu, F. G. West, J. Org. Chem. 2010, 75, 5410–5413;
- 3dW. T. Spencer, M. D. Levin, A. J. Frontier, Org. Lett. 2011, 13, 414–417;
- 3eZ.-X. Ma, S. He, W. Song, R. P. Hsung, Org. Lett. 2012, 14, 5736–5739.
- 4Review: T. N. Grant, C. J. Rieder, F. G. West, Chem. Commun. 2009, 5676–5688.
- 5
- 5aF. Dhoro, T. E. Kristensen, V. Stockmann, G. P. A. Yap, M. A. Tius, J. Am. Chem. Soc. 2007, 129, 7256–7257;
- 5bD. Song, A. Rostami, F. G. West, J. Am. Chem. Soc. 2007, 129, 12019–12022;
- 5cA. Rostami, Y. Wang, A. M. Arif, R. McDonald, F. G. West, Org. Lett. 2007, 9, 703–706;
- 5dV. M. Marx, D. J. Burnell, Org. Lett. 2009, 11, 1229–1231.
- 6An important exception is the 1,2-Wagner–Meerwein shift of alkyl and aryl groups in certain highly substituted substrates:
- 6aJ. Huang, D. Leboeuf, A. J. Frontier, J. Am. Chem. Soc. 2011, 133, 6307–6317;
- 6bD. Leboeuf, V. Gandon, J. Ciesielski, A. J. Frontier, J. Am. Chem. Soc. 2012, 134, 6296–6308.
- 7M. Oishi, H. Takikawa in Science of Synthesis Knowledge Updates 2010, 93–112.
- 8
- 8aJ. D. Rainier, J. M. Cox, Org. Lett. 2000, 2, 2707–2709;
- 8bK. Haraguchi, Y. Kubota, H. Tanaka, J. Org. Chem. 2004, 69, 1831–1836.
- 9
- 9aY. Kitagawa, S. Hashimoto, S. Iemura, H. Yamamoto, H. Nozaki, J. Am. Chem. Soc. 1976, 98, 5030–5031;
- 9bS. Flemming, J. Kabbara, K. Nickisch, J. Westermann, J. Mohr, Synlett 1995, 183–185.
- 10K. Ishihara, N. Hanaki, H. Yamamoto, J. Am. Chem. Soc. 1993, 115, 10695–10704.
- 11Use of EtAlCl2 as Lewis acid in Nazarov reaction:
- 11aG. Liang, S. N. Gradl, D. Trauner, Org. Lett. 2003, 5, 4931–4934;
- 11bS.-H. Kim, J. K. Cha, Synthesis 2000, 2113–2116;
- 11cFor a recent example of R3Al-initiated Nazarov cyclization followed by trapping with pendent phosphine, see: J. Boudreau, M.-A. Courtemanche, V. M. Marx, D. J. Burnell, F.-G. Fontaine, Chem. Commun. 2012, 48, 11250–11252.
- 12Use of Me2AlCl in 6π electrocyclization: L. M. Bishop, J. E. Barbarow, R. G. Bergman, D. Trauner, Angew. Chem. 2008, 120, 8220–8223; Angew. Chem. Int. Ed. 2008, 47, 8100–8103.
- 13Halide trapping of the Nazarov intermediate has been postulated to occur by TiIV-mediated delivery of halogen ligands:
- 13aT. D. White, F. G. West, Tetrahedron Lett. 2005, 46, 5629–5632;
- 13bV. M. Marx, T. S. Cameron, D. J. Burnell, Tetrahedron Lett. 2009, 50, 7213–7216.
- 14Triorganoaluminum reagents are highly sensitive to traces of water, and inclusion of molecular sieves in the reaction mixture was found to improve reproducibility.
- 15CCDC 915117 (3 a), 915118 (3 h), 915119 (7 a), 915120 (8 a′), and 915121 (11 a) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 16While we have occasionally observed competing 1,4-addition of nucleophilic traps to Lewis acid-activated dienones, simple 1,2-addition has not been observed previously.
- 17For an alternative approach to the “reductive Nazarov” reaction, see:
- 17aS. Giese, F. G. West, Tetrahedron 2000, 56, 10221–10228;
- 17bS. Giese, F. G. West, Tetrahedron Lett. 1998, 39, 8393–8396.
- 18K. Ziegler, H. Martin, F. Krupp, Justus Liebigs Ann. Chem. 1960, 629, 14–19.
- 19Consistent chemical shift differences for methyl groups cis and trans to adjacent phenyl groups have been observed in this study and in previous cases. See reference [17a] for an example.
- 20
- 20aA. K. Basak, M. A. Tius, Org. Lett. 2008, 10, 4073–4076;
- 20bC. J. Rieder, R. J. Fradette, F. G. West, Heterocycles 2010, 80, 1413–1427.
- 21O. Scadeng, M. J. Ferguson, F. G. West, Org. Lett. 2011, 13, 114–117.
- 22A. G. Schultz, M. Macielag, M. Plummer, J. Org. Chem. 1988, 53, 391–395.
- 23V. Aureggi, G. Sedelmeier, Angew. Chem. 2007, 119, 8592–8596;
10.1002/ange.200701045 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8440–8444.
- 24Catalytic amounts of DBU were added to an NMR tube containing 11 a′ and the reaction was monitored by 1H NMR spectroscopy over 15 h. During this time, 40 % conversion into 11 a was observed.