N-Heterocyclic Carbene Catalyzed [4+3] Annulation of Enals and o-Quinone Methides: Highly Enantioselective Synthesis of Benzo-ε-Lactones†
Prof. Dr. Hui Lv
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190 (China)
Key Laboratory for Green Chemical Process of Ministry of Education, School of material science and Engineering, Wuhan Institute of Technology, Wuhan, 430073 (China)
Search for more papers by this authorWen-Qiang Jia
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190 (China)
Search for more papers by this authorLi-Hui Sun
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Song Ye
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190 (China)
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190 (China)Search for more papers by this authorProf. Dr. Hui Lv
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190 (China)
Key Laboratory for Green Chemical Process of Ministry of Education, School of material science and Engineering, Wuhan Institute of Technology, Wuhan, 430073 (China)
Search for more papers by this authorWen-Qiang Jia
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190 (China)
Search for more papers by this authorLi-Hui Sun
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Song Ye
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190 (China)
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190 (China)Search for more papers by this authorFinancial support from the Ministry of Science and Technology of China (2011CB808600), National Natural Science Foundation of China (No. 21072195, 20932008), and the Chinese Academy of Sciences is gratefully acknowledged.
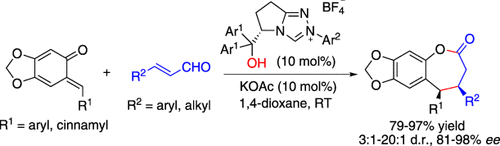
Graphical Abstract
Enantioselectivity through H bonding: An unprecedented [4+3] annulation of enals with o-quinone methides catalyzed by N-heterocyclic carbenes (NHCs) to give benzo-ε-lactones is described. High to excellent enantioselectivity was achieved by using a chiral triazolium NHC having a free OH group, which participates in a hydrogen-bonding interaction with the substrate.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303903_sm_miscellaneous_information.pdf3.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected examples, see:
- 1aV. Lukaćova, J. Polonsky, C. Moretti, G. R. Pettit, J. M. Schmidt, J. Nat. Prod. 1982, 45, 288;
- 1bZ. Lidert, D. A. H. Taylor, M. Thirugnanam, J. Nat. Prod. 1985, 48, 843;
- 1cL. Rodríguez-Hahn, J. Cárdenas, C. Arenas, Phytochemistry 1996, 43, 457;
- 1dF. R. Garcez, W. S. Garcez, M. T. Tsutsumi, N. F. Roque, Phytochemistry 1997, 45, 141;
- 1eX.-D. Luo, S.-H. Wu, D.-G. Wu, Y.-B. Ma, S.-H. Qi, Tetrahedron 2002, 58, 7797;
- 1fO. Koul, W. M. Daniewski, J. S. Multani, M. Gumulka, G. Singh, J. Agric. Food Chem. 2003, 51, 7271;
- 1gX.-H. Cai, Z.-Z. Du, X.-D. Luo, Helv. Chim. Acta 2007, 90, 1980;
- 1hB.-D. Lin, H.-D. Chen, J. Liu, S. Zhang, Y. Wu, L. Dong, J.-M. Yue, Phytochemistry 2010, 71, 1596;
- 1iS.-P. Yang, H.-D. Chen, S.-G. Liao, B.-J. Xie, Z.-H. Miao, J.-M. Yue, Org. Lett. 2011, 13, 150;
- 1jY. Zhang, J.-S. Wang, X.-B. Wang, Y.-C. Gu, D.-D. Wei, C. Guo, M.-H. Yang, L.-Y. Kong, J. Agric. Food Chem. 2013, 61, 2171.
- 2
- 2aG. Peris, S. J. Miller, Org. Lett. 2008, 10, 3049;
- 2bA. Rioz-Martínez, G. de Gonzalo, D. E. T. Pazmiño, M. W. Fraaije, V. Gotor, Eur. J. Org. Chem. 2009, 2526;
- 2cM. Uyanik, D. Nakashima, K. Ishihara, Angew. Chem. 2012, 124, 9227;
10.1002/ange.201204286 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 9093.
- 3For the enantioselective synthesis of ε-lactones by Bayer–Villiger reaction, see:
- 3aM. T. Reetz, B. Brunner, T. Schneider, F. Schulz, C. M. Clouthier, M. M. Kayser, Angew. Chem. 2004, 116, 4167;
10.1002/ange.200460272 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 4075;
- 3bM. D. Mihovilovic, F. Rudroff, B. Grötzl, P. Kapitan, R. Snajdrova, J. Rydz, R. Mach, Angew. Chem. 2005, 117, 3675;
10.1002/ange.200462964 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3609;
- 3cF. Hollmann, A. Taglieber, F. Schulz, M. T. Reetz, Angew. Chem. 2007, 119, 2961;
10.1002/ange.200605169 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 2903;
- 3dM. T. Reetz, S. Wu, J. Am. Chem. Soc. 2009, 131, 15424;
- 3eL. Zhou, X. Liu, J. Ji, Y. Zhang, X. Hu, L. Lin, X. Feng, J. Am. Chem. Soc. 2012, 134, 17023.
- 4M. Murakami, T. Tsuruta, Y. Ito, Angew. Chem. 2000, 112, 2600;
Angew. Chem. Int. Ed. 2000, 39, 2484.
10.1002/1521-3773(20000717)39:14<2484::AID-ANIE2484>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 5
- 5aB. El Ali, K. Okuro, G. Vasapollo, H. Alper, J. Am. Chem. Soc. 1996, 118, 4264;
- 5bR. Chanthateyanonth, H. Alper, Adv. Synth. Catal. 2004, 346, 1375.
- 6
- 6aL. Wang, K. Thai, M. Gravel, Org. Lett. 2009, 11, 891;
- 6bG.-Q. Li, Y. Li, L.-X. Dai, S.-L. You, Org. Lett. 2007, 9, 3519.
- 7For reviews on NHC catalysis, see:
- 7aD. Enders, T. Balensiefer, Acc. Chem. Res. 2004, 37, 534;
- 7bD. Enders, O. Niemeier, A. Henseler, Chem. Rev. 2007, 107, 5606;
- 7cV. Nair, S. Vellalath, B. P. Babu, Chem. Soc. Rev. 2008, 37, 2691;
- 7dJ. L. Moore, T. Rovis, Top. Curr. Chem. 2010, 291, 77;
- 7eV. Nair, R. S. Menon, A. T. Biju, C. R. Sinu, R. R. Paul, Chem. Soc. Rev. 2011, 40, 5336;
- 7fA. Grossmann, D. Enders, Angew. Chem. 2012, 124, 320; Angew. Chem. Int. Ed. 2012, 51, 314.
- 8
- 8aS. S. Sohn, E. L. Rosen, J. W. Bode, J. Am. Chem. Soc. 2004, 126, 14370;
- 8bC. Burstein, F. Glorius, Angew. Chem. 2004, 116, 6331;
10.1002/ange.200461572 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 6205;
- 8cV. Nair, S. Vellalath, M. Poonoth, R. Mohan, E. Suresh, Org. Lett. 2006, 8, 507;
- 8dK. Hirano, I. Piel, F. Glorius, Adv. Synth. Catal. 2008, 350, 984;
- 8eY. Li, Z.-A. Zhao, H. He, S.-L. You, Adv. Synth. Catal. 2008, 350, 1885;
- 8fL.-H. Sun, L.-T. Shen, S. Ye, Chem. Commun. 2011, 47, 10136.
- 9
- 9aM. He, J. W. Bode, Org. Lett. 2005, 7, 3131;
- 9bD. E. A. Raup, B. Cardinal-David, D. Holte, K. A. Scheidt, Nat. Chem. 2010, 2, 766;
- 9cX. Zhao, D. A. DiRocco, T. Rovis, J. Am. Chem. Soc. 2011, 133, 12466;
- 9dM. Rommel, T. Fukuzumi, J. W. Bode, J. Am. Chem. Soc. 2008, 130, 17266;
- 9eP. Zheng, C. A. Gondo, J. W. Bode, Chem. Asian J. 2011, 6, 614;
- 9fB. Zhang, P. Feng, L.-H. Sun, Y. Cui, S. Ye, N. Jiao, Chem. Eur. J. 2012, 18, 9198;
- 9gH. Lv, B. Tiwari, J. Mo, C. Xing, Y. R. Chi, Org. Lett. 2012, 14, 5412; For other related NHC-catalyzed reaction of enals, see:
- 9hY. Wu, W. Yao, L. Pan, Y. Zhang, C. Ma, Org. Lett. 2010, 12, 640;
- 9iL. Yang, B. Tan, F. Wang, G. Zhong, J. Org. Chem. 2009, 74, 1744.
- 10
- 10aA. Chan, K. A. Scheidt, J. Am. Chem. Soc. 2007, 129, 5334;
- 10bE. M. Phillips, T. E. Reynolds, K. A. Scheidt, J. Am. Chem. Soc. 2008, 130, 2416.
- 11For the formal [3+2] annulation, see:
- 11aV. Nair, S. Vellalath, M. Poonoth, E. Suresh, J. Am. Chem. Soc. 2006, 128, 8736;
- 11bP.-C. Chiang, J. Kaeobamrung, J. W. Bode, J. Am. Chem. Soc. 2007, 129, 3520;
- 11cJ. Kaeobamrung, J. W. Bode, Org. Lett. 2009, 11, 677;
- 11dV. Nair, R. R. Paul, D. V. M. Padmaja, N. Aiswarya, C. R. Sinu, A. Jose, Tetrahedron 2011, 67, 9885;
- 11eM. He, J. W. Bode, J. Am. Chem. Soc. 2008, 130, 418.
- 12For the [4+2] annulation, see:
- 12aM. He, G. J. Uc, J. W. Bode, J. Am. Chem. Soc. 2006, 128, 15088;
- 12bM. He, B. J. Beahm, J. W. Bode, Org. Lett. 2008, 10, 3817;
- 12cJ. Kaeobamrung, M. C. Kozlowski, J. W. Bode, Proc. Natl. Acad. Sci. USA 2010, 107, 20661;
- 12dV. Nair, R. R. Paul, K. C. S. Lakshmi, R. S. Menon, A. Jose, C. R. Sinu, Tetrahedron Lett. 2011, 52, 5992;
- 12eX. Fang, X. Chen, Y. R. Chi, Org. Lett. 2011, 13, 4708;
- 12fZ. Fu, H. Sun, S. Chen, B. Tiwari, G. Li, Y. R. Chi, Chem. Commun. 2013, 49, 261.
- 13M. He, J. R. Struble, J. W. Bode, J. Am. Chem. Soc. 2006, 128, 8418.
- 14
- 14aS. De Sarkar, A. Studer, Angew. Chem. 2010, 122, 9452;
10.1002/ange.201004593 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 9266;
- 14bZ.-Q. Rong, M.-Q. Jia, S.-L. You, Org. Lett. 2011, 13, 4080.
- 15For NHC-catalyzed annulation reaction of substrates other than enals, see:
- 15aF.-G. Sun, L.-H. Sun, S. Ye, Adv. Synth. Catal. 2011, 353, 3134;
- 15bC. Yao, D. Wang, J. Lu, T. Li, W. Jiao, C. Yu, Chem. Eur. J. 2012, 18, 1914;
- 15cJ. Kaeobamrung, J. Mahatthananchai, P. Zheng, J. W. Bode, J. Am. Chem. Soc. 2010, 132, 8810;
- 15dZ.-Q. Zhu, J.-C. Xiao, Adv. Synth. Catal. 2010, 352, 2455;
- 15eS. J. Ryan, L. Candish, D. W. Lupton, J. Am. Chem. Soc. 2009, 131, 14176;
- 15fC. Awasthi, L. D. S. Yadav, Synlett 2010, 1783;
- 15gR. L. Atienza, H. S. Roth, K. A. Scheidt, Chem. Sci. 2011, 2, 1772;
- 15hS. J. Ryan, L. Candish, D. W. Lupton, J. Am. Chem. Soc. 2011, 133, 4694;
- 15iL. Hao, Y. Du, H. Lv, X. Chen, H. Jiang, Y. Shao, Y. R. Chi, Org. Lett. 2012, 14, 2154.
- 16
- 16aY.-R. Zhang, L. He, X. Wu, P.-L. Shao, S. Ye, Org. Lett. 2008, 10, 277;
- 16bX.-N. Wang, P.-L. Shao, H. Lv, S. Ye, Org. Lett. 2009, 11, 4029;
- 16cX.-N. Wang, Y.-R. Zhang, S. Ye, Adv. Synth. Catal. 2010, 352, 1892;
- 16dP.-L. Shao, X.-Y. Chen, S. Ye, Angew. Chem. 2010, 122, 8590; Angew. Chem. Int. Ed. 2010, 49, 8412;
- 16eT.-Y. Jian, L. He, C. Tang, S. Ye, Angew. Chem. 2011, 123, 9270; Angew. Chem. Int. Ed. 2011, 50, 9104; Smith et al. also independently established similar reactions:
- 16fN. Duguet, C. D. Campbell, A. M. Z. Slawin, A. D. Smith, Org. Biomol. Chem. 2008, 6, 1108;
- 16gN. Duguet, A. Donaldson, S. M. Leckie, E. A. Kallström, C. D. Campbell, P. Shapland, T. B. Brown, A. M. Z. Slawin, A. D. Smith, Tetrahedron: Asymmetry 2010, 21, 601.
- 17H. Lv, L. You, S. Ye, Adv. Synth. Catal. 2009, 351, 2822.
- 18
- 18aE. Alden-Danforth, M. T. Scerba, T. Lectka, Org. Lett. 2008, 10, 4951;
- 18bR. W. Van De Water, T. R. R. Pettus, Tetrahedron 2002, 58, 5367.
- 19Nair et al. reported an NHC-catalyzed [8+3] annulation of enals and tropone: V. Nair, M. Poonoth, S. Vellalath, E. Suresh, R. Thirumalai, J. Org. Chem. 2006, 71, 8964.
- 20
- 20aD. Enders, O. Niemeier, T. Balensiefer, Angew. Chem. 2006, 118, 1491;
10.1002/ange.200503885 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 1463;
- 20bD. Enders, J. Han, Tetrahedron: Asymmetry 2008, 19, 1367;
- 20cD. Enders, J. Han, Synthesis 2008, 3864;
- 20dD. Enders, J. Han, A. Henseler, Chem. Commun. 2008, 3989.
- 21
- 21aL. He, Y.-R. Zhang, X.-L. Huang, S. Ye, Synthesis 2008, 2825;
- 21bX.-L. Huang, L. He, P.-L. Shao, S. Ye, Angew. Chem. 2009, 121, 198; Angew. Chem. Int. Ed. 2009, 48, 192;
- 21cX.-L. Huang, S. Ye, Chin. Sci. Bull. 2010, 55, 1753;
- 21dL. Baragwanath, C. A. Rose, K. Zeitler, S. J. Connon, J. Org. Chem. 2009, 74, 9214.
- 22L. H. Sun, Z.-Q. Liang, W.-Q. Jia, S. Ye, Angew. Chem. 2013, 125, 5915; Angew. Chem. Int. Ed. 2013, 52, 5803.
- 23CCDC 937173 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. See supporting information for the Figure S1.
- 24The absolute stereochemistry of product 3 is preliminarily assigned by the comparison to a previous related reaction (Ref [8f]).
- 25A. Chan, K. A. Scheidt, J. Am. Chem. Soc. 2006, 128, 4558.
- 26No desired product 3 was observed for the NHC-catalyzed reaction of the correponding phenol of quinone methide 1 a and bromoenal or ynal.
- 27We have become aware of a related study by Scheidt and co-workers involving the formal [4+3] annulation of NHC-homoenolates and o-quinone methides generated in situ. K. Scheidt, personal communication.