Release and Recovery of Guest Molecules during the Reversible Borate Gel Formation of Guest-Included Macrocyclic Boronic Esters†
Dr. Suguru Ito
Department of Chemistry, Tokyo Institute of Technology, and JST-CREST, 2-12-1, O-okayama, Meguro-ku, Tokyo 152-8551 (Japan)
Search for more papers by this authorHisatsugu Takata
Department of Chemistry, Tokyo Institute of Technology, and JST-CREST, 2-12-1, O-okayama, Meguro-ku, Tokyo 152-8551 (Japan)
Search for more papers by this authorDr. Kosuke Ono
Department of Chemistry, Tokyo Institute of Technology, and JST-CREST, 2-12-1, O-okayama, Meguro-ku, Tokyo 152-8551 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Nobuharu Iwasawa
Department of Chemistry, Tokyo Institute of Technology, and JST-CREST, 2-12-1, O-okayama, Meguro-ku, Tokyo 152-8551 (Japan)
Department of Chemistry, Tokyo Institute of Technology, and JST-CREST, 2-12-1, O-okayama, Meguro-ku, Tokyo 152-8551 (Japan)Search for more papers by this authorDr. Suguru Ito
Department of Chemistry, Tokyo Institute of Technology, and JST-CREST, 2-12-1, O-okayama, Meguro-ku, Tokyo 152-8551 (Japan)
Search for more papers by this authorHisatsugu Takata
Department of Chemistry, Tokyo Institute of Technology, and JST-CREST, 2-12-1, O-okayama, Meguro-ku, Tokyo 152-8551 (Japan)
Search for more papers by this authorDr. Kosuke Ono
Department of Chemistry, Tokyo Institute of Technology, and JST-CREST, 2-12-1, O-okayama, Meguro-ku, Tokyo 152-8551 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Nobuharu Iwasawa
Department of Chemistry, Tokyo Institute of Technology, and JST-CREST, 2-12-1, O-okayama, Meguro-ku, Tokyo 152-8551 (Japan)
Department of Chemistry, Tokyo Institute of Technology, and JST-CREST, 2-12-1, O-okayama, Meguro-ku, Tokyo 152-8551 (Japan)Search for more papers by this authorThanks are due to Dr. Hidehiro Uekusa and Dr. Kohei Johmoto for performing X-ray analysis. This work was supported by a CREST (Core Research for Evolutional Science and Technology) project from the Japan Science and Technology Agency (JST).
Graphical Abstract
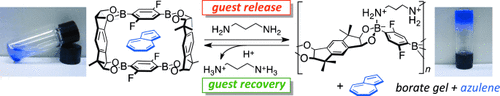
Borate gel formation from guest-encapsulated macrocyclic boronic esters was realized by the addition of a diamine to the suspension of the boronic esters in various organic solvents, which triggered the release of the guest compounds. The guest molecules could be recovered from the borate gel by addition of an acid to remove the diamine, which facilitated the reconstruction of the initial guest-encapsulated macrocyclic boronic esters.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303870_sm_miscellaneous_information.pdf8.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. N. H. Reek, S. Otto, Dynamic Combinatorial Chemistry, Wiley-VCH, Weinheim, 2010.
10.1002/9783527629701 Google Scholar
- 2For reviews, see:
- 2aP. T. Corbett, J. Leclaire, L. Vial, K. R. West, J.-L. Wietor, J. K. M. Sanders, S. Otto, Chem. Rev. 2006, 106, 3652–3711;
- 2bN. E. Borisova, M. D. Reshetova, Y. A. Ustynyuk, Chem. Rev. 2007, 107, 46–79;
- 2cM. Mastalerz, Angew. Chem. 2010, 122, 5164–5175;
10.1002/ange.201000443 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 5042–5053;
- 2dR. Nishiyabu, Y. Kubo, T. D. James, J. S. Fossey, Chem. Commun. 2011, 47, 1124–1150.
- 3For reviews, see:
- 3aR. W. Saalfrank, H. Maid, A. Scheurer, Angew. Chem. 2008, 120, 8924–8956; Angew. Chem. Int. Ed. 2008, 47, 8794–8824;
- 3bM. J. Wiester, P. A. Ulmann, C. A. Mirkin, Angew. Chem. 2011, 123, 118–142; Angew. Chem. Int. Ed. 2011, 50, 114–137;
- 3cR. Chakrabarty, P. S. Mukherjee, P. J. Stang, Chem. Rev. 2011, 111, 6810–6918.
- 4For reviews, see:
- 4aR. J. Wojtecki, M. A. Meador, S. J. Rowan, Nat. Mater. 2011, 10, 14–27;
- 4bX. Yan, F. Wang, B. Zheng, F. Huang, Chem. Soc. Rev. 2012, 41, 6042–6065;
- 4cL. E. Buerkle, S. J. Rowan, Chem. Soc. Rev. 2012, 41, 6089–6102;
- 4dC. Tomasini, N. Castellucci, Chem. Soc. Rev. 2013, 42, 156–172.
- 5For some recent examples of the smart gel based on the dynamic covalent bond formation, see:
- 5aN. Sreenivasachary, J.-M. Lehn, Proc. Natl. Acad. Sci. USA 2005, 102, 5938–5943;
- 5bE. Buhler, N. Sreenivasachary, S.-J. Candau, J.-M. Lehn, J. Am. Chem. Soc. 2007, 129, 10058–10059;
- 5cN. Sreenivasachary, J.-M. Lehn, Chem. Asian J. 2008, 3, 134–139;
- 5dA. P. Vogt, B. S. Sumerlin, Soft Matter 2009, 5, 2347–2351;
- 5eG. Deng, C. Tang, F. Li, H. Jiang, Y. Chen, Macromolecules 2010, 43, 1191–1194;
- 5fY. Amamoto, J. Kamada, H. Otsuka, A. Takahara, K. Matyjaszewski, Angew. Chem. 2011, 123, 1698–1701; Angew. Chem. Int. Ed. 2011, 50, 1660–1663;
- 5gX. de Hatten, N. Bell, N. Yufa, G. Christmann, J. R. Nitschke, J. Am. Chem. Soc. 2011, 133, 3158–3164;
- 5hJ. Li, J. M. A. Carnall, M. C. A. Stuart, S. Otto, Angew. Chem. 2011, 123, 8534–8536; Angew. Chem. Int. Ed. 2011, 50, 8384–8386;
- 5iK. Imato, M. Nishihara, T. Kanehara, Y. Amamoto, A. Takahara, H. Otsuka, Angew. Chem. 2012, 124, 1164–1168;
10.1002/ange.201104069 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 1138–1142.
- 6For some recent examples of the smart gel based on the reversible metal-ligand coordination, see:
- 6aS. Ray, A. K. Das, A. Banerjee, Chem. Mater. 2007, 19, 1633–1639;
- 6bS. Zhang, S. Yang, J. Lan, Y. Tang, Y. Xue, J. You, J. Am. Chem. Soc. 2009, 131, 1689–1691;
- 6cM.-O. M. Piepenbrock, N. Clarke, J. W. Steed, Soft Matter 2010, 6, 3541–3547;
- 6dH. Lee, J. H. Lee, S. Kang, J. Y. Lee, G. John, J. H. Jung, Chem. Commun. 2011, 47, 2937–2939;
- 6eT. D. Hamilton, D.-K. Bučar, J. Baltrusaitis, D. R. Flanagan, Y. Li, S. Ghorai, A. V. Tivanski, L. R. MacGillivray, J. Am. Chem. Soc. 2011, 133, 3365–3371;
- 6fW. J. Gee, S. R. Batten, Chem. Commun. 2012, 48, 4830–4832.
- 7Relatively weak interactions (hydrogen bonding, π-stacking, van der Waals interactions, etc.) have also been utilized for the formation of smart gels. For some recent examples, see:
- 7aX. Chen, Z. Huang, S.-Y. Chen, K. Li, X.-Q. Yu, L. Pu, J. Am. Chem. Soc. 2010, 132, 7297–7299;
- 7bM. Ikeda, T. Tanida, T. Yoshii, I. Hamachi, Adv. Mater. 2011, 23, 2819–2822;
- 7cC. A. Strassert, C.-H. Chien, M. D. G. Lopez, D. Kourkoulos, D. Hertel, K. Meerholz, L. D. Cola, Angew. Chem. 2011, 123, 976–980; Angew. Chem. Int. Ed. 2011, 50, 946–950;
- 7dT. Tu, W. Fang, X. Bao, X. Li, K. H. Dötz, Angew. Chem. 2011, 123, 6731–6735; Angew. Chem. Int. Ed. 2011, 50, 6601–6605;
- 7eY. Li, T. Park, J. K. Quansah, S. C. Zimmerman, J. Am. Chem. Soc. 2011, 133, 17118–17121;
- 7fO. Kotova, R. Daly, C. M. G. dos Santos, M. Boese, P. E. Kruger, J. J. Boland, T. Gunnlaugsson, Angew. Chem. 2012, 124, 7320–7324;
10.1002/ange.201201506 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 7208–7212;
- 7gA. Dawn, T. Shiraki, H. Ichikawa, A. Takada, Y. Takahashi, Y. Tsuchiya, L. T. N. Lien, S. Shinkai, J. Am. Chem. Soc. 2012, 134, 2161–2171.
- 8
- 8aP. K. Vemula, J. Li, G. John, J. Am. Chem. Soc. 2006, 128, 8932–8938;
- 8bB. Adhikari, G. Palui, A. Banerjee, Soft Matter 2009, 5, 3452–3460;
- 8cG. Liang, Z. Yang, R. Zhang, L. Li, Y. Fan, Y. Kuang, Y. Gao, T. Wang, W. W. Lu, B. Xu, Langmuir 2009, 25, 8419–8422;
- 8dQ. Chen, Y. Lv, D. Zhang, G. Zhang, C. Liu, D. Zhu, Langmuir 2010, 26, 3165–3168;
- 8eX. Li, J. Li, Y. Gao, Y. Kuang, J. Shi, B. Xu, J. Am. Chem. Soc. 2010, 132, 17707–17709;
- 8fJ. Boekhoven, M. Koot, T. A. Wezendonk, R. Eelkema, J. H. van Esch, J. Am. Chem. Soc. 2012, 134, 12908–12911.
- 9For reviews, see:
- 9aM. D. Pluth, K. N. Raymond, Chem. Soc. Rev. 2007, 36, 161–171;
- 9bH.-J. Schneider, Angew. Chem. 2009, 121, 3982–4036;
10.1002/ange.200802947 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 3924–3977;
- 9cS. Rieth, K. Hermann, B.-Y. Wang, J. D. Badjić, Chem. Soc. Rev. 2011, 40, 1609–1622;
- 9dG. H. Clever in Molecules at Work (Ed.: ), Wiley-VCH, Weinheim, 2012, pp. 13–37.
10.1002/9783527645787.ch2 Google Scholar
- 10Host–guest interactions of a gelator has only been utilized for the formation of supramolecular polymer gel. For a review, see:
- 10aJ. A. Foster, J. W. Steed, Angew. Chem. 2010, 122, 6868–6874; Angew. Chem. Int. Ed. 2010, 49, 6718–6724; For some recent examples, see:
- 10bE. A. Appel, F. Biedermann, U. Rauwald, S. T. Jones, J. M. Zayed, O. A. Scherman, J. Am. Chem. Soc. 2010, 132, 14251–14260;
- 10cS. Dong, Y. Luo, X. Yan, B. Zheng, X. Ding, Y. Yu, Z. Ma, Q. Zhao, F. Huang, Angew. Chem. 2011, 123, 1945–1949; Angew. Chem. Int. Ed. 2011, 50, 1905–1909;
- 10dM. Zhang, D. Xu, X. Yan, J. Chen, S. Dong, B. Zheng, F. Huang, Angew. Chem. 2012, 124, 7117–7121; Angew. Chem. Int. Ed. 2012, 51, 7011–7015.
- 11
- 11aN. Iwasawa, H. Takahagi, J. Am. Chem. Soc. 2007, 129, 7754–7755;
- 11bH. Takahagi, S. Fujibe, N. Iwasawa, Chem. Eur. J. 2009, 15, 13327–13330;
- 11cH. Takahagi, N. Iwasawa, Chem. Eur. J. 2010, 16, 13680–13688.
- 12
- 12aD. M. Schubert in Group 13 Chemistry III, Industrial Applications, Vol. 105 (Eds.: ), Structure and Bonding Series, Springer, Berlin, 2003, pp. 1–40;
- 12bE. Galbraith, T. D. James, Chem. Soc. Rev. 2010, 39, 3831–3842;
- 12cR. Nishiyabu, Y. Kubo, T. D. James, J. S. Fossey, Chem. Commun. 2011, 47, 1106–1123.
- 13To the best of our knowledge, there has been only one report that utilizes reversible borate formation for the creation of supramolecular gel. In this example, the borate network was obtained from 4-tert-butylcatechol ester of a triboronic acid with 4,4′-bipyridine. See: E. Sheepwash, V. Krampl, R. Scopelliti, O. Sereda, A. Neels, K. Severin, Angew. Chem. 2011, 123, 3090–3093; Angew. Chem. Int. Ed. 2011, 50, 3034–3037.
- 14CCDC 936722 ([2+2]⋅toluene) and 936723 ([2+2]⋅naphthalene) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15T. Kishida, N. Fujita, K. Sada, S. Shinkai, J. Am. Chem. Soc. 2005, 127, 7298–7299.
- 16M. Zhang, L. Meng, X. Cao, M. Jiang, T. Yi, Soft Matter 2012, 8, 4494–4498.
- 17Solvents used for the gelation did not affect the chemical structure of the borate gel. Dried gel obtained by suction filtration and vacuum drying of the borate gel prepared in methanol/toluene (2:1) also exhibited almost the same 11B NMR signal in the solid-state NMR, indicating the existence of the borate structure (see the Supporting Information, Figure S7).
- 18
- 18aG. Ferguson, J. F. Gallagher, D. Murphy, J. P. Sheehan, T. R. Spalding, Polyhedron 1993, 12, 859–864;
- 18bA. Abreu, S. J. Alas, H. I. Beltrán, R. Santillan, N. Farfán, J. Organomet. Chem. 2006, 691, 337–348.
- 19No oligomeric species were observed by 1H NMR analysis when [2+2]⋅toluene was added to the mixture of CuCl2 with 1,3-diaminopropane in CDCl3 and filtered after standing at room temperature for 12 h. Therefore, the mixture, initially released from the xerogel by the addition of CuCl2 in CDCl3, should represent the components of boronic ester moieties in the xerogel.
- 20When 1 M aqueous HCl was added to the borate gel prepared in dioxane or benzene, oligomeric boronic esters were obtained as precipitate along with [2+2], indicating that the self-assembly of [2+2] did not occur efficiently in the absence of methanol which facilitates the equilibrium of the boronic ester. This result also supported that the formation of borate gel is caused by the cross-linking of oligomeric boronic esters and [2+2] by the diamine.
- 21J. Yan, G. Springsteen, S. Deeter, B. Wang, Tetrahedron 2004, 60, 11205–11209.