Palladium-Catalyzed Asymmetric Intermolecular Cyclization†
Dr. Jian Hu
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorProf. Dr. Hajime Hirao
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorDr. Yongxin Li
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jianrong (Steve) Zhou
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)Search for more papers by this authorDr. Jian Hu
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorProf. Dr. Hajime Hirao
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorDr. Yongxin Li
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jianrong (Steve) Zhou
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)Search for more papers by this authorWe thank the Singapore National Research Foundation (NRF-RF2008-10) and Nanyang Technological University for financial support and Sijia Liu for some experiments.
Graphical Abstract
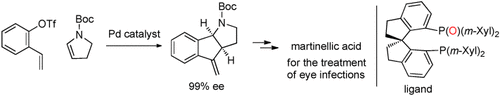
Domino cyclization: Alkylpalladium intermediates in an asymmetric Heck reaction were intercepted by a second alkene to give tricyclic products with high enantioselectivity (see scheme; Boc=tert-butoxycarbonyl). The method was applied to the asymmetric synthesis of a precursor of (−)-martinellic acid, a folk eye medicine in South America.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303753_sm_miscellaneous_information.pdf9.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aO. Loiseleur, M. Hayashi, M. Keenan, N. Schmees, A. Pfaltz, J. Organomet. Chem. 1999, 576, 16;
- 1bL. F. Tietze, H. Ila, H. P. Bell, Chem. Rev. 2004, 104, 3453;
- 1cD. Mc Cartney, P. J. Guiry, Chem. Soc. Rev. 2011, 40, 5122;
- 1dM. Shibasaki, E. M. Vogl, T. Ohshima, Adv. Synth. Catal. 2004, 346, 1533; for an asymmetric Heck reaction of acyclic olefins with aryl diazonium salts, see:
- 1e E. W. Werner, T.-S. Mei, A. J. Burckle, M. S. Sigman, Science 2012, 338, 1455.
- 2
- 2aF. Ozawa, A. Kubo, T. Hayashi, J. Am. Chem. Soc. 1991, 113, 1417;
- 2bF. Ozawa, Y. Kobatake, T. Hayashi, Tetrahedron Lett. 1993, 34, 2505.
- 3For examples, see:
- 3aS. R. Gilbertson, Z. Fu, Org. Lett. 2001, 3, 161;
- 3bW. J. Drury, N. Zimmermann, M. Keenan, M. Hayashi, S. Kaiser, R. Goddard, A. Pfaltz, Angew. Chem. 2004, 116, 72;
10.1002/ange.200352755 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 70;
- 3cY. Mata, O. Pàmies, M. Diéguez, Chem. Eur. J. 2007, 13, 3296;
- 3dJ. Mazuela, O. Pàmies, M. Diéguez, Chem. Eur. J. 2010, 16, 3434;
- 3eW.-Q. Wu, Q. Peng, D.-X. Dong, X.-L. Hou, Y.-D. Wu, J. Am. Chem. Soc. 2008, 130, 9717;
- 3fL.-X. Dai, T. Tu, S.-L. You, W.-P. Deng, X.-L. Hou, Acc. Chem. Res. 2003, 36, 659;
- 3gM. O. Fitzpatrick, H. Muller-Bunz, P. J. Guiry, Eur. J. Org. Chem. 2009, 1889;
- 3hM. Rubina, W. M. Sherrill, M. Rubin, Organometallics 2008, 27, 6393;
- 3iS. T. Henriksen, P.-O. Norrby, P. Kaukoranta, P. G. Andersson, J. Am. Chem. Soc. 2008, 130, 10414;
- 3jK. Yonehara, K. Mori, T. Hashizume, K.-G. Chung, K. Ohe, S. Uemura, J. Organomet. Chem. 2000, 603, 40;
- 3kL. F. Tietze, K. Thede, F. Sannicolo, Chem. Commun. 1999, 1811;
- 3lN. G. Andersen, M. Parvez, B. A. Keay, Org. Lett. 2000, 2, 2817;
- 3mG. Trabesinger, A. Albinati, N. Feiken, R. W. Kunz, P. S. Pregosin, M. Tschoerner, J. Am. Chem. Soc. 1997, 119, 6315.
- 4
- 4aO. Loiseleur, P. Meier, A. Pfaltz, Angew. Chem. 1996, 108, 218;
10.1002/ange.19961080223 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 200;
- 4bO. Loiseleur, M. Hayashi, N. Schmees, A. Pfaltz, Synthesis 1997, 1338.
- 5For reviews, see:
- 5aG. Poli, G. Giambastiani, A. Heumann, Tetrahedron 2000, 56, 5959;
- 5bA. de Meijere, S. Bräse, J. Organomet. Chem. 1999, 576, 88;
- 5cA. Heumann, M. Réglier, Tetrahedron 1996, 52, 9289;
- 5dL. F. Tietze, Chem. Rev. 1996, 96, 115;
- 5eE.-i. Negishi, C. Copéret, S. Ma, S.-Y. Liou, F. Liu, Chem. Rev. 1996, 96, 365;
- 5fO. René, D. Lapointe, K. Fagnou, Org. Lett. 2009, 11, 4560.
- 6S. Bräse, J. Rümper, K. Voigt, S. Albecq, G. Thurau, R. Villard, B. Waegell, A. de Meijere, Eur. J. Org. Chem. 1998, 671.
10.1002/(SICI)1099-0690(199804)1998:4<671::AID-EJOC671>3.0.CO;2-R CAS Web of Science® Google Scholar
- 7T. H. Wöste, M. Oestreich, Chem. Eur. J. 2011, 17, 11914.
- 8
- 8aJ.-H. Xie, Q.-L. Zhou, Acc. Chem. Res. 2008, 41, 581;
- 8b(R)-Xyl-SDP(O) can be synthesized from the R diol or purchased from Zonebanner Jiuzhou Co., Zhejiang, China.
- 9I. D. Hills, G. C. Fu, J. Am. Chem. Soc. 2004, 126, 13178.
- 10
- 10aK. K. Hii, T. D. W. Claridge, J. M. Brown, Angew. Chem. 1997, 109, 1033;
10.1002/ange.19971090925 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 984;
- 10bB. M. M. Wheatley, B. A. Keay, J. Org. Chem. 2007, 72, 7253.
- 11Examples:
- 11aX. Wu, J. Zhou, Chem. Commun. 2013, 49, 4794;
- 11bM. Catellani, G. P. Chiusoli, S. Ricotti, J. Organomet. Chem. 1985, 296, c 11;
- 11cO. Reiser, M. Weber, A. de Meijere, Angew. Chem. 1989, 101, 1071; Angew. Chem. Int. Ed. Engl. 1989, 28, 1037;
- 11dB. Mariampillai, J. Alliot, M. Li, M. Lautens, J. Am. Chem. Soc. 2007, 129, 15372.
- 12CCDC 932284 and 932283 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 13K. M. Witherup, R. W. Ransom, A. C. Graham, A. M. Bernard, M. J. Salvatore, W. C. Lumma, P. S. Anderson, S. M. Pitzenberger, S. L. Varga, J. Am. Chem. Soc. 1995, 117, 6682.
- 14Examples:
- 14aD. Ma, C. Xia, J. Jiang, J. Zhang, Org. Lett. 2001, 3, 2189;
- 14bB. B. Snider, Y. Ahn, S. M. O’Hare, Org. Lett. 2001, 3, 4217;
- 14cS. Ikeda, M. Shibuya, Y. Iwabuchi, Chem. Commun. 2007, 504;
- 14dB.-L. Lei, C.-H. Ding, X.-F. Yang, X.-L. Wan, X.-L. Hou, J. Am. Chem. Soc. 2009, 131, 18250;
- 14eS. G. Davies, A. M. Fletcher, J. A. Lee, T. J. A. Lorkin, P. M. Roberts, J. E. Thomson, Org. Lett. 2013, 15, 2050.
- 15M. Ueda, S. Kawai, M. Hayashi, T. Naito, O. Miyata, J. Org. Chem. 2010, 75, 914.
- 16R. F. Borch, M. D. Bernstein, H. D. Durst, J. Am. Chem. Soc. 1971, 93, 2897.
- 171,1′-Spirobiindane-7,7′-bisphosphane oxides can form highly active catalysts of palladium for simple asymmetric Heck reactions of cyclic olefins: J. Hu, H. Hirao, Y. Li, J. Zhou, unpublished results.