NHC Organocatalytic Formal LUMO Activation of α,β-Unsaturated Esters for Reaction with Enamides†
Dr. Jiajia Cheng
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore) http://www.ntu.edu.sg/home/robinchi
Search for more papers by this authorZhijian Huang
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore) http://www.ntu.edu.sg/home/robinchi
Search for more papers by this authorCorresponding Author
Prof. Dr. Yonggui Robin Chi
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore) http://www.ntu.edu.sg/home/robinchi
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore) http://www.ntu.edu.sg/home/robinchiSearch for more papers by this authorDr. Jiajia Cheng
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore) http://www.ntu.edu.sg/home/robinchi
Search for more papers by this authorZhijian Huang
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore) http://www.ntu.edu.sg/home/robinchi
Search for more papers by this authorCorresponding Author
Prof. Dr. Yonggui Robin Chi
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore) http://www.ntu.edu.sg/home/robinchi
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore) http://www.ntu.edu.sg/home/robinchiSearch for more papers by this authorWe thank the Singapore National Research Foundation (NRF), the Singapore Economic Development Board (EDB), GlaxoSmithKline (GSK), and Nanyang Technological University (NTU) for generous financial support, and Dr. Y. Li and Dr. R. Ganguly (NTU) for X-ray crystal-structure analysis. LUMO=lowest unoccupied molecular orbital, NHC=N-heterocyclic carbene.
Graphical Abstract
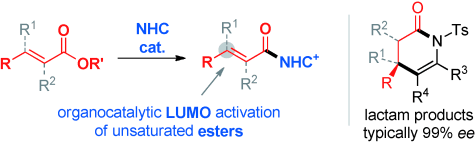
An effective wake-up call: Stable α,β-unsaturated esters were activated by the addition of a chiral N-heterocyclic carbene (NHC) organocatalyst, and the resulting reactive Michael acceptor intermediates reacted with enamide nucleophiles to furnish optically pure products (see scheme; Ts=p-toluenesulfonyl). These products can be converted readily into bioactive δ-lactams, piperidines, and their derivatives.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303247_sm_miscellaneous_information.pdf9.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see:
- 1aM. Shibasaki, M. Kanai, Chem. Rev. 2008, 108, 2853;
- 1bJ. M. Janey, Angew. Chem. 2005, 117, 4364;
10.1002/ange.200462314 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 4292;
- 1cB. Weiner, W. Szymański, D. B. Janssen, A. J. Minnaard, B. L. Feringa, Chem. Soc. Rev. 2010, 39, 1656;
- 1dG. P. Howell, Org. Process Res. Dev. 2012, 16, 1258.
- 2For selected examples, see:
- 2aJ. A. Kalow, A. G. Doyle, J. Am. Chem. Soc. 2010, 132, 3268;
- 2bZ. Xu, L. Liu, K. Wheeler, H. Wang, Angew. Chem. 2011, 123, 3546; Angew. Chem. Int. Ed. 2011, 50, 3484;
- 2cT. Hashimoto, Y. Naganawa, K. Maruoka, J. Am. Chem. Soc. 2011, 133, 8834;
- 2dH. Suga, Y. Furihata, A. Sakamoto, K. Itoh, Y. Okumura, T. Tsuchida, A. Kakehi, T. Baba, J. Org. Chem. 2011, 76, 7377;
- 2eC. Schotes, M. Althaus, R. Aardoom, A. Mezzetti, J. Am. Chem. Soc. 2012, 134, 1331;
- 2fK. Shibatomi, Y. Soga, A. Narayama, I. Fujisawa, S. Iwasa, J. Am. Chem. Soc. 2012, 134, 9836.
- 3
- 3aD. A. Evans, K. A. Woerpel, M. M. Hinman, M. M. Faul, J. Am. Chem. Soc. 1991, 113, 726;
- 3bE. J. Corey, N. Imai, H.-Y. Zhang, J. Am. Chem. Soc. 1991, 113, 728;
- 3cJ. F. Johnson, D. A. Evans, Acc. Chem. Res. 2000, 33, 325;
- 3dG. Desimoni, G. Faita, K. A. Jørgensen, Chem. Rev. 2011, 111, PR 284.
- 4M. Shibasaki, M. Kanai, S. Matsunaga, N. Kumagai, Acc. Chem. Res. 2009, 42, 1117.
- 5D. H. Paull, C. J. Abraham, M. T. Scerba, E. Alden-Danforth, T. Lectka, Acc. Chem. Res. 2008, 41, 655.
- 6X. Liu, L. Lin, X. Feng, Acc. Chem. Res. 2011, 44, 574.
- 7
- 7aK. A. Ahrendt, C. J. Borths, D. W. C. MacMillan, J. Am. Chem. Soc. 2000, 122, 4243; for selected reviews, see:
- 7bA. Erkkilä, I. Majander, P. M. Pihko, Chem. Rev. 2007, 107, 5416;
- 7cP. Melchiorre, M. Marigo, A. Carlone, G. Bartoli, Angew. Chem. 2008, 120, 6232;
10.1002/ange.200705523 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6138;
- 7dS. Bertelsen, K. A. Jørgensen, Chem. Soc. Rev. 2009, 38, 2178;
- 7eD. Enders, C. Wang, J. X. Liebich, Chem. Eur. J. 2009, 15, 11058;
- 7fJ.-L. Li, T.-Y. Liu, Y.-C. Chen, Acc. Chem. Res. 2012, 45, 1491;
- 7gJ. Alemán, C. Silvia, Chem. Soc. Rev. 2013, 42, 774.
- 8For selected examples and reviews of enamine/iminium catalysis, see:
- 8aB. List, R. A. Lerner, C. F. Barbas III, J. Am. Chem. Soc. 2000, 122, 2395;
- 8bB. List, P. Pojarliev, W. T. Biller, H. J. Martin, J. Am. Chem. Soc. 2002, 124, 827;
- 8cY. Hayashi, H. Gotoh, T. Hayashi, M. Shoji, Angew. Chem. 2005, 117, 4284; Angew. Chem. Int. Ed. 2005, 44, 4212;
- 8dJ. Franzén, M. Marigo, D. Fielenbach, T. C. Wabnitz, A. Kjærsgaard, K. A. Jørgensen, J. Am. Chem. Soc. 2005, 127, 18296;
- 8eD. Enders, M. R. M. Hüttl, C. Grondal, G. Raabe, Nature 2006, 441, 861;
- 8fA. Moyano, R. Rios, Chem. Rev. 2011, 111, 4703.
- 9
- 9aG. A. Grasa, R. M. Kissling, S. P. Nolan, Org. Lett. 2002, 4, 3583;
- 9bG. W. Nyce, J. A. Lamboy, E. F. Connor, R. M. Waymouth, J. L. Hedrick, Org. Lett. 2002, 4, 3587;
- 9cM. Movassaghi, M. A. Schmidt, Org. Lett. 2005, 7, 2453;
- 9dC.-L. Lai, H. M. Lee, C.-H. Hu, Tetrahedron Lett. 2005, 46, 6265; for other relevant studies, see:
- 9eR. C. Samanta, S. De Sarkar, R. Fröhlich, S. Grimme, A. Studer, Chem. Sci. 2013, 4, 2177.
- 10L. Hao, Y. Du, H. Lv, X. Chen, H. Jiang, Y. Shao, Y. R. Chi, Org. Lett. 2012, 14, 2154.
- 11For selected reviews on NHC catalysis, see:
- 11aK. Zeitler, Angew. Chem. 2005, 117, 7674;
10.1002/ange.200502617 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 7506;
- 11bD. Enders, O. Niemeier, A. Henseler, Chem. Rev. 2007, 107, 5606;
- 11cN. Marion, S. Díez-González, S. P. Nolan, Angew. Chem. 2007, 119, 3046;
10.1002/ange.200603380 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 2988;
- 11dV. Nair, S. Vellalath, B. P. Babu, Chem. Soc. Rev. 2008, 37, 2691;
- 11eA. J. Arduengo III, L. I. Iconaru, Dalton Trans. 2009, 6903;
- 11fE. M. Phillips, A. Chan, K. A. Scheidt, Aldrichimica Acta 2009, 42, 55;
- 11gJ. L. Moore, T. Rovis, Top. Curr. Chem. 2010, 291, 77;
- 11hA. T. Biju, N. Kuhl, F. Glorius, Acc. Chem. Res. 2011, 44, 1182;
- 11iK. Hirano, I. Piel, F. Glorius, Chem. Lett. 2011, 40, 786;
- 11jP.-C. Chiang, J. W. Bode, TCI MAIL 2011, 149, 2;
- 11kV. Nair, R. S. Menon, A. T. Biju, C. R. Sinu, R. R. Paul, A. Jose, V. Sreekumar, Chem. Soc. Rev. 2011, 40, 5336;
- 11lZ. Q. Rong, W. Zhang, G. Q. Yang, S.-L. You, Curr. Org. Chem. 2011, 15, 3077;
- 11mH. U. Vora, T. Rovis, Aldrichimica Acta 2011, 44, 3;
- 11nD. T. Cohen, K. A. Scheidt, Chem. Sci. 2012, 3, 53;
- 11oX. Bugaut, F. Glorius, Chem. Soc. Rev. 2012, 41, 3511;
- 11pA. Grossmann, D. Enders, Angew. Chem. 2012, 124, 320; Angew. Chem. Int. Ed. 2012, 51, 314;
- 11qJ. Douglas, G. Churchill, A. D. Smith, Synthesis 2012, 2295;
- 11rJ. Izquierdo, G. E. Hutson, D. T. Cohen, K. A. Scheidt, Angew. Chem. 2012, 124, 11854;
10.1002/ange.201203704 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 11686.
- 12
- 12aB. E. Maki, A. Chan, E. M. Phillips, K. A. Scheidt, Org. Lett. 2007, 9, 371;
- 12bS. De Sarkar, S. Grimme, A. Studer, J. Am. Chem. Soc. 2010, 132, 1190;
- 12cS. De Sarkar, A. Studer, Angew. Chem. 2010, 122, 9452;
10.1002/ange.201004593 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 9266;
- 12dR. S. Reddy, J. N. Rosa, L. F. Veiros, S. Caddick, P. M. P. Gois, Org. Biomol. Chem. 2011, 9, 3126;
- 12eZ.-Q. Rong, M.-Q. Jia, S.-L. You, Org. Lett. 2011, 13, 4080;
- 12fA. Biswas, S. De Sarkar, L. Tebben, A. Studer, Chem. Commun. 2012, 48, 5190;
- 12gY. Lu, W. Tang, Y. Zhang, D. Du, T. Lu, Adv. Synth. Catal. 2013, 355, 321;
- 12hS. Lu, S. B. Poh, W.-Y. Siau, Y. Zhao, Angew. Chem. 2013, 125, 1775; Angew. Chem. Int. Ed. 2013, 52, 1731.
- 13B. Wanner, J. Mahatthananchai, J. W. Bode, Org. Lett. 2011, 13, 5378.
- 14
- 14aF.-G. Sun, L.-H. Sun, S. Ye, Adv. Synth. Catal. 2011, 353, 3134;
- 14bC. Yao, D. Wang, J. Lu, T. Li, W. Jiao, C. Yu, Chem. Eur. J. 2012, 18, 1914;
- 14cB. Zhang, P. Feng, Y. Cui, N. Jiao, Chem. Commun. 2012, 48, 7280;
- 14dS. R. Yetra, A. Bhunia, A. Patra, M. V. Mane, K. Vanka, A. T. Biju, Adv. Synth. Catal. 2013, 355, 1089.
- 15
- 15aK. Zeitler, Org. Lett. 2006, 8, 637;
- 15bJ. Kaeobamrung, J. Mahatthananchai, P. Zheng, J. W. Bode, J. Am. Chem. Soc. 2010, 132, 8810;
- 15cZ.-Q. Zhu, X.-L. Zheng, N.-F. Jiang, X. Wan, J.-C. Xiao, Chem. Commun. 2011, 47, 8670;
- 15dJ. Mahatthananchai, P. Zheng, J. W. Bode, Angew. Chem. 2011, 123, 1711; Angew. Chem. Int. Ed. 2011, 50, 1673;
- 15eD. Du, Z. Hu, J. Jin, Y. Lu, W. Tang, B. Wang, T. Lu, Org. Lett. 2012, 14, 1274;
- 15fF. Romanov-Michailidis, C. Besnard, A. Alexakis, Org. Lett. 2012, 14, 4906.
- 16
- 16aS. J. Ryan, L. Candish, D. W. Lupton, J. Am. Chem. Soc. 2011, 133, 4694;
- 16bL. Candish, D. W. Lupton, J. Am. Chem. Soc. 2013, 135, 58.
- 17
- 17aS. J. Ryan, L. Candish, D. W. Lupton, J. Am. Chem. Soc. 2009, 131, 14176;
- 17bL. Candish, D. W. Lupton, Org. Biomol. Chem. 2011, 9, 8182;
- 17cL. Candish, D. W. Lupton, Chem. Sci. 2012, 3, 380;
- 17dS. J. Ryan, L. Candish, D. W. Lupton, Chem. Soc. Rev. 2013, 42, 4906.
- 18E. R. T. Robinson, C. Fallan, C. Simal, A. M. Z. Slawin, A. D. Smith, Chem. Sci. 2013, 4, 2193.
- 19M. S. Kharasch, B. S. Joshi, J. Org. Chem. 1957, 22, 1439.
- 20For the use of enamides derived from 1,3-dicarbonyl compounds and cyclic enamides as nucleophiles, see:
- 20aA. G. Kravina, J. Mahatthananchai, J. W. Bode, Angew. Chem. 2012, 124, 9568;
10.1002/ange.201204145 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 9433 and Ref. [12]; for the use of enamines derived from cyclic ketones as nucleophiles, see:
- 20bR. C. Samanta, B. Maji, S. De Sarkar, K. Bergander, R. Fröhlich, C. Mück-Lichtenfeld, H. Mayr, A. Studer, Angew. Chem. 2012, 124, 5325; Angew. Chem. Int. Ed. 2012, 51, 5234.
- 21
- 21aR. L. Knight, F. J. Leeper, J. Chem. Soc. Perkin Trans. 1 1998, 1891;
- 21bD. Enders, U. Kallfass, Angew. Chem. 2002, 114, 1822;
10.1002/1521-3757(20020517)114:10<1822::AID-ANGE1822>3.0.CO;2-W Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1743;10.1002/1521-3773(20020517)41:10<1743::AID-ANIE1743>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 21cM. S. Kerr, J. Read de Alaniz, T. Rovis, J. Am. Chem. Soc. 2002, 124, 10298;
- 21dH. Takikawa, Y. Hachisu, J. W. Bode, K. Suzuki, Angew. Chem. 2006, 118, 3572;
10.1002/ange.200600268 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 3492;
- 21eJ. R. Struble, J. W. Bode, Org. Synth. 2010, 87, 362.
- 22
- 22aM. Wadamoto, E. M. Phillips, T. E. Reynolds, K. A. Scheidt, J. Am. Chem. Soc. 2007, 129, 10098;
- 22bT. Jousseaume, N. E. Wurz, F. Glorius, Angew. Chem. 2011, 123, 1446; Angew. Chem. Int. Ed. 2011, 50, 1410.
- 23CCDC 932918 (3 c) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 24For a discussion of the mechanism of the Claisen-type pathway, see Ref. [14b]; see also:
- 24aJ. Mahatthananchai, J. Kaeobamrung, J. W. Bode, ACS Catal. 2012, 2, 494;
- 24bE. Lyngvi, J. W. Bode, F. Schoenebeck, Chem. Sci. 2012, 3, 2346.
- 25C. F. Timponi, N. E. Oliveira, R. M. P. Arruda, S. S. Meyrelles, E. C. Vasquez, Basic Clin. Pharmacol. Toxicol. 2006, 98, 518.
- 26
- 26aP. L. Beaulieu, D. Wernic, J. Org. Chem. 1996, 61, 3635;
- 26bM. R. Reeder, R. M. Anderson, Chem. Rev. 2006, 106, 2828.
- 27B. E. Lewis, D. E. Wallis, S. D. Berkowitz, W. H. Matthai, J. Fareed, J. M. Walenga, J. Bartholomew, R. Sham, R. G. Lerner, Z. R. Zeigler, P. K. Rustagi, I. K. Jang, S. D. Rifkin, J. Moran, M. J. Hursting, J. G. Kelton, Circulation 2001, 103, 1838.
- 28K. Suzuki, T. Sato, M. Morioka, K. Nagai, K. Abe, H. Yamaguchi, T. Saito, Y. Ohmi, K. Susaki, J. Antibiot. 1991, 44, 479.
- 29
- 29aV. Uzunova, Y. Sheline, J. M. Davis, A. Rasmusson, D. P. Uzunov, E. Costa, A. Guidotti, Proc. Natl. Acad. Sci. USA 1998, 95, 3239;
- 29bC. Hiemke, S. Härtter, Pharmacol. Ther. 2000, 85, 11.
- 30C. Simal, T. Lebl, A. M. Z. Slawin, A. D. Smith, Angew. Chem. 2012, 124, 3713;
10.1002/ange.201109061 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 3653.
- 31B. Han, J.-L. Li, C. Ma, S.-J. Zhang, Y.-C. Chen, Angew. Chem. 2008, 120, 9929; Angew. Chem. Int. Ed. 2008, 47, 9971.