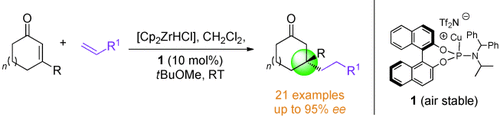Formation of Quaternary Centers by Copper-Catalyzed Asymmetric Conjugate Addition of Alkylzirconium Reagents†
Dr. Mireia Sidera
Department of Chemistry, Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA (UK)
These authors contributed equally to this work.
Search for more papers by this authorPhilippe M. C. Roth
Department of Chemistry, Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA (UK)
These authors contributed equally to this work.
Search for more papers by this authorRebecca M. Maksymowicz
Department of Chemistry, Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA (UK)
Search for more papers by this authorCorresponding Author
Dr. Stephen P. Fletcher
Department of Chemistry, Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA (UK)
Department of Chemistry, Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA (UK)Search for more papers by this authorDr. Mireia Sidera
Department of Chemistry, Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA (UK)
These authors contributed equally to this work.
Search for more papers by this authorPhilippe M. C. Roth
Department of Chemistry, Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA (UK)
These authors contributed equally to this work.
Search for more papers by this authorRebecca M. Maksymowicz
Department of Chemistry, Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA (UK)
Search for more papers by this authorCorresponding Author
Dr. Stephen P. Fletcher
Department of Chemistry, Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA (UK)
Department of Chemistry, Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA (UK)Search for more papers by this authorThe authors thank the EPSRC for generous support of this research in the form of a Career Acceleration Fellowship to S.F. (EP/H003711/1). We are grateful to Prof. A. Alexakis for GC analysis of 3 b and Dr. B. Odell for assistance with the NMR experiments.
Graphical Abstract
Pure and simple: Alkylzirconocenes generated in situ from simple alkenes are used in highly enantioselective copper-catalyzed 1,4-addition reactions to trisubstituted cyclic enones to generate quaternary centers. These reactions operate at room temperature under a range of conditions and tolerate many functional groups. Cp=cyclopentadienyl, Tf=trifluoromethanesulfonyl.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303202_sm_miscellaneous_information.pdf3.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. Patai, The Chemistry of the Double Bonded Functional Groups, Wiley, Chichester, UK, 1997.
10.1002/0470857234 Google Scholar
- 2
- 2aR. M. Maksymowicz, P. M. C. Roth, S. P. Fletcher, Nat. Chem. 2012, 4, 649–654;
- 2bR. M. Maksymowicz, P. M. C. Roth, A. L. Thompson, S. P. Fletcher, Chem. Commun. 2013, 49, 4211–4213.
- 3
- 3aJ. Christoffers, G. Koripelly, A. Rosiak, M. Rössle, Synthesis 2007, 1279–1300;
- 3bT. Jerphagnon, M. G. Pizzuti, A. J. Minnaard, B. L. Feringa, Chem. Soc. Rev. 2009, 38, 1039–1075;
- 3cA. Alexakis, J. E. Backvall, N. Krause, O. Pamies, M. Dieguez, Chem. Rev. 2008, 108, 2796–2823;
- 3dT. Thaler, P. Knochel, Angew. Chem. 2009, 121, 655–658;
10.1002/ange.200804446 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 645–648.
- 4
- 4aJ. Schwartz, J. A. Labinger, Angew. Chem. 1976, 88, 402–409; Angew. Chem. Int. Ed. Engl. 1976, 15, 333–340;
- 4bS. L. Buchwald, S. J. LaMaire, R. B. Nielsen, Org. Synth. 1993, 71, 77–82.
- 5P. Wipf, H. Jahn, Tetrahedron 1996, 52, 12853–12910.
- 6
- 6aJ. P. Das, I. Marek, Chem. Commun. 2011, 47, 4593–4623;
- 6bB. M. Trost, C. H. Jiang, Synthesis 2006, 369–396;
- 6cC. J. Douglas, L. E. Overman, Proc. Natl. Acad. Sci. USA 2004, 101, 5363–5367;
- 6dJ. Christoffers, A. Baro, Angew. Chem. 2003, 115, 1726–1728;
10.1002/ange.200201614 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1688–1690;
- 6eE. J. Corey, A. Guzman-Perez, Angew. Chem. 1998, 110, 2092–2118;
10.1002/(SICI)1521-3757(19980803)110:15<2092::AID-ANGE2092>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 1998, 37, 388–401.10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V PubMed Web of Science® Google Scholar
- 7C. Hawner, A. Alexakis, Chem. Commun. 2010, 46, 7295–7306.
- 8
- 8aJ. Wu, D. M. Mampreian, A. H. Hoveyda, J. Am. Chem. Soc. 2005, 127, 4584–4585;
- 8bA. W. Hird, A. H. Hoveyda, J. Am. Chem. Soc. 2005, 127, 14988–14989;
- 8cK. S. Lee, M. K. Brown, A. W. Hird, A. H. Hoveyda, J. Am. Chem. Soc. 2006, 128, 7182–7184;
- 8dE. Fillion, A. Wilsily, J. Am. Chem. Soc. 2006, 128, 2774–2775;
- 8eM. K. Brown, T. L. May, C. A. Baxter, A. H. Hoveyda, Angew. Chem. 2007, 119, 1115–1118; Angew. Chem. Int. Ed. 2007, 46, 1097–1100;
- 8fA. M. Dumas, E. Fillion, Acc. Chem. Res. 2010, 43, 440–454.
- 9
- 9aM. d’Augustin, L. Palais, A. Alexakis, Angew. Chem. 2005, 117, 1400–1402;
10.1002/ange.200462137 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1376–1378;
- 9bL. Palais, I. S. Mikhel, C. Bournaud, L. Micouin, C. A. Falciola, M. Vuagnoux-d’Augustin, S. Rosset, G. Bernardinelli, A. Alexakis, Angew. Chem. 2007, 119, 7606–7609;
10.1002/ange.200702186 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 7462–7465;
- 9cM. Vuagnoux-d′Augustin, A. Alexakis, Chem. Eur. J. 2007, 13, 9647–9662;
- 9dM. K. Brown, A. H. Hoveyda, J. Am. Chem. Soc. 2008, 130, 12904–12906;
- 9eT. L. May, M. K. Brown, A. H. Hoveyda, Angew. Chem. 2008, 120, 7468–7472; Angew. Chem. Int. Ed. 2008, 47, 7358–7362;
- 9fL. Palais, A. Alexakis, Chem. Eur. J. 2009, 15, 10473–10485;
- 9gA. Mendoza, Y. Ishihara, P. S. Baran, Nat. Chem. 2012, 4, 21–25.
- 10
- 10aD. Martin, S. Kehrli, M. d’Augustin, H. Clavier, M. Mauduit, A. Alexakis, J. Am. Chem. Soc. 2006, 128, 8416–8417;
- 10bH. Hénon, M. Mauduit, A. Alexakis, Angew. Chem. 2008, 120, 9262–9264;
10.1002/ange.200803735 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 9122–9124;
- 10cY. Matsumoto, K. I. Yamada, K. Tomioka, J. Org. Chem. 2008, 73, 4578–4581;
- 10dS. Kehrli, D. Martin, D. Rix, M. Mauduit, A. Alexakis, Chem. Eur. J. 2010, 16, 9890–9904;
- 10eN. Germain, M. Magrez, S. Kehrli, M. Mauduit, A. Alexakis, Eur. J. Org. Chem. 2012, 5301–5306;
- 10fM. Tissot, D. Poggiali, H. Henon, D. Muller, L. Guenee, M. Mauduit, A. Alexakis, Chem. Eur. J. 2012, 18, 8731–8747;
- 10gJ. Y. W. Mak, C. M. Williams, Chem. Commun. 2012, 48, 287–289.
- 11G. P. Howell, Org. Process Res. Dev. 2012, 16, 1258–1272.
- 12S. R. Harutyunyan, T. den Hartog, K. Geurts, A. J. Minnaard, B. L. Feringa, Chem. Rev. 2008, 108, 2824–2852.
- 13J. F. Teichert, B. L. Feringa, Angew. Chem. 2010, 122, 2538–2582;
10.1002/ange.200904948 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 2486–2528.
- 14Compound (R)-3 m was converted in three steps into (R)-8 which has known and meaningful optical rotation. See the Supporting Information.

- 15
- 15aM. Vuagnoux-d’Augustin, S. Kehrli, A. Alexakis, Synlett 2007, 2057–2060;
- 15bC. Hawner, K. Y. Li, V. Cirriez, A. Alexakis, Angew. Chem. 2008, 120, 8334–8337;
10.1002/ange.200803436 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 8211–8214.