Reversible Switching of the Luminescence of a Photoresponsive Gadolinium(III) Complex†
Corresponding Author
Prof. Hidetaka Nakai
Department of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395 (Japan)
Department of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395 (Japan)Search for more papers by this authorKazuhiro Kitagawa
Department of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395 (Japan)
Search for more papers by this authorHarutaka Nakamori
Department of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395 (Japan)
Search for more papers by this authorTaisuke Tokunaga
Department of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395 (Japan)
Search for more papers by this authorDr. Takahiro Matsumoto
Department of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395 (Japan)
International Institute for Carbon-Neutral Energy Research (WPI-I2CNER), Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395 (Japan)
Search for more papers by this authorProf. Koichi Nozaki
Department of Chemistry, Graduate School of Science & Engineering, University of Toyama, 3190 Gofuku, Toyama 930-8555 (Japan)
Search for more papers by this authorProf. Seiji Ogo
Department of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395 (Japan)
International Institute for Carbon-Neutral Energy Research (WPI-I2CNER), Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395 (Japan)
Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Agency (JST), Kawaguchi Center Building, 4-1-8 Honcho Kawaguchi-shi Saitama 332-0012 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Hidetaka Nakai
Department of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395 (Japan)
Department of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395 (Japan)Search for more papers by this authorKazuhiro Kitagawa
Department of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395 (Japan)
Search for more papers by this authorHarutaka Nakamori
Department of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395 (Japan)
Search for more papers by this authorTaisuke Tokunaga
Department of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395 (Japan)
Search for more papers by this authorDr. Takahiro Matsumoto
Department of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395 (Japan)
International Institute for Carbon-Neutral Energy Research (WPI-I2CNER), Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395 (Japan)
Search for more papers by this authorProf. Koichi Nozaki
Department of Chemistry, Graduate School of Science & Engineering, University of Toyama, 3190 Gofuku, Toyama 930-8555 (Japan)
Search for more papers by this authorProf. Seiji Ogo
Department of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395 (Japan)
International Institute for Carbon-Neutral Energy Research (WPI-I2CNER), Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395 (Japan)
Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Agency (JST), Kawaguchi Center Building, 4-1-8 Honcho Kawaguchi-shi Saitama 332-0012 (Japan)
Search for more papers by this authorThis work was supported by grants-in-aid: 23655054 and 24109016 (Scientific Research on Innovative Areas “Stimuli-responsive Chemical Species”) and the World Premier International Research Center Initiative (WPI Program) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) (Japan) and the Basic Research Programs CREST Type “Development of the Foundation for Nano-Interface Technology” from JST (Japan).
Graphical Abstract
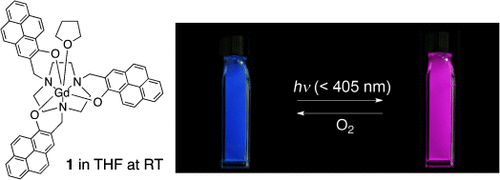
From blue to red: The structure and luminescence properties of the gadolinium(III) complex 1 were investigated. Reversible switching of the luminescence of 1 in THF at room temperature by alternating light irradiation and O2 exposure is presented, during which the emission color changes as shown in the picture. Light-induced phosphorescence of 1 plays a key role in this behavior.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303137_sm_miscellaneous_information.pdf4.9 MB | miscellaneous_information |
| anie_201303137_sm_movie_s1.mpg5 MB | movie_s1 |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aP. Bamfield, M. G. Hutchings, Chromic Phenomena: Technological Applications of Colour Chemistry, 2nd ed., RSC Publishing, Cambridge, 2010;
- 1bH. Dürr, H. Bouas-Laurent, Photochromism: Molecules and Systems, Elsevier, Amsterdam, 2003;
- 1cSpecial edition: Photochromism: Memories and Switches (Ed.: M. Irie), Chem. Rev. 2000, 100.
- 2
- 2aS. Chen, L.-J. Chen, H.-B. Yang, H. Tian, W. Zhu, J. Am. Chem. Soc. 2012, 134, 13596;
- 2bJ. C.-H. Chan, W. H. Lam, H.-L. Wong, N. Zhu, W.-T. Wong, V. W.-W. Yam, J. Am. Chem. Soc. 2011, 133, 12690;
- 2cJ.-K. Sun, L.-X. Cai, Y.-J. Chen, Z.-H. Li, J. Zhang, Chem. Commun. 2011, 47, 6870;
- 2dJ. Kärnbratt, M. Hammarson, S. Li, H. L. Anderson, B. Albinsson, J. Andréasson, Angew. Chem. 2010, 122, 1898;
10.1002/ange.200906088 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 1854;
- 2eT. Nakagawa, Y. Hasegawa, T. Kawai, Chem. Commun. 2009, 5630;
- 2fM. Morimoto, H. Miyasaka, M. Yamashita, M. Irie, J. Am. Chem. Soc. 2009, 131, 9823.
- 3
- 3aT. Ishizuka, T. Sawaki, S. Miyazaki, M. Kawano, Y. Shiota, K. Yoshizawa, S. Fukuzumi, T. Kojima, Chem. Eur. J. 2011, 17, 6652;
- 3bB. A. McClure, E. R. Abrams, J. J. Rack, J. Am. Chem. Soc. 2010, 132, 5428;
- 3cH. Nakai, T. Nonaka, Y. Miyano, M. Mizuno, Y. Ozawa, K. Toriumi, N. Koga, T. Nishioka, M. Irie, K. Isobe, J. Am. Chem. Soc. 2008, 130, 17836.
- 4
- 4aM. Akita, Organometallics 2011, 30, 43;
- 4bY. Hasegawa, T. Nakagawa, T. Kawai, Coord. Chem. Rev. 2010, 254, 2643;
- 4cV. Guerchais, L. Ordronneau, H. L. Bozec, Coord. Chem. Rev. 2010, 254, 2533;
- 4dS. Kume, H. Nishihara, Struct. Bonding (Berlin) 2007, 123, 79.
- 5
- 5aH. Nakai, K. Isobe, Coord. Chem. Rev. 2010, 254, 2652;
- 5bJ. G. Vos, M. T. Pryce, Coord. Chem. Rev. 2010, 254, 2519;
- 5c Inorganic Chromotropism: Basic Concepts and Applications of Colored Materials (Ed.: ), Kodansha/Springer, Tokyo, 2007.
- 61,4,7-Triazacyclononane (tacn=C6H15N3) derivatives have been successfully utilized as powerful ligands in f-element chemistry. See:
- 6aP. Benndorf, S. Schmitt, R. Köppe, P. Oña-Burgos, A. Scheurer, K. Meyer, P. W. Roesky, Angew. Chem. 2012, 124, 5091;
10.1002/ange.201109109 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 5006;
- 6bA. Nonat, D. Imbert, J. Pécaut, M. Giraud, M. Mazzanti, Inorg. Chem. 2009, 48, 4207;
- 6cI. Castro-Rodríguez, K. Meyer, Chem. Commun. 2006, 1353;
- 6dA. Nonat, C. Gateau, P. H. Fries, M. Mazzanti, Chem. Eur. J. 2006, 12, 7133;
- 6eH. Nakai, X. Hu, L. N. Zakharov, A. L. Rheingold, K. Meyer, Inorg. Chem. 2004, 43, 855;
- 6fI. Castro-Rodríguez, K. Olsen, P. Gantzel, K. Meyer, J. Am. Chem. Soc. 2003, 125, 4565;
- 6gV. Alexander, Chem. Rev. 1995, 95, 273;
- 6hL. Prodi, M. Maestri, R. Ziessel, V. Balzani, Inorg. Chem. 1991, 30, 3798.
- 7In this paper, the irradiation wavelength (λirr) is used for photochromism. The excitation wavelength (λex) is used for photoluminescence. In each experiment, the λirr and λex were used as indicated in each Figure caption.
- 8
- 8aA. Strasser, A. Vogler, J. Photochem. Photobiol. A 2004, 165, 115;
- 8bG. Knör, A. Strasser, Inorg. Chem. Commun. 2002, 5, 993;
- 8cJ. R. Shaw, R. H. Schmehl, J. Am. Chem. Soc. 1991, 113, 389.
- 9A. Vogler, H. Kunkely, Inorg. Chim. Acta 2006, 359, 4130.
- 10The aryloxide-functionalized tacn derivatives can be synthesized through Mannich reactions: P. Chaudhuri, K. Wieghardt in Progress in Inorganic Chemistry, Vol. 50 (Ed.: ), Wiley, New York, 2002, pp. 151–216. Also see: Ref. [6e, f].
- 11T. Yatabe, H. Nakai, K. Nozaki, T. Yamamura, K. Isobe, Organometallics 2010, 29, 2390.
- 12The emission lifetime of the fluorescence is below the measurement limit of the equipment (<50 μs).
- 13The gadolinium(III) complexes scarcely show the emission from MC (metal-centered) ff, MC fd, MLCT (metal-to-ligand charge transfer), LMCT (ligand-to-metal charge transfer), and MMCT (metal-to-metal charge transfer) excited states, because the Gd3+ ion is highly stabilized by its half-filled f shell: the emission from the intraligand excited states is usually observed. See, Refs. [6h, 9] and [11].
- 14A. Robertson, Nature 1948, 162, 153.
- 15Recently, the O2-quenching behavior of phosphorescent materials has become appealing for its potential application to optical oxygen sensing. See:
- 15aC. S. Smith, K. R. Mann, J. Am. Chem. Soc. 2012, 134, 8786;
- 15bX. Liu, W. Sun, L. Zou, Z. Xie, X. Li, C. Lu, L. Wang, Y. Cheng, Dalton Trans. 2012, 41, 1312;
- 15cD. E. Achatz, R. J. Meier, L. H. Fischer, O. S. Wolfbeis, Angew. Chem. 2011, 123, 274; Angew. Chem. Int. Ed. 2011, 50, 260.
- 16
- 16aP. Caravan, Acc. Chem. Res. 2009, 42, 851;
- 16bM. Bottrill, L. Kwok, N. J. Long, Chem. Soc. Rev. 2006, 35, 557;
- 16cP. Caravan, Chem. Soc. Rev. 2006, 35, 512;
- 16dP. Caravan, J. J. Ellison, T. J. McMurry, R. B. Lauffer, Chem. Rev. 1999, 99, 2293.
- 17
- 17aX.-J. Zhu, T. Zhang, S. Zhao, W.-K. Wong, W.-Y. Wong, Eur. J. Inorg. Chem. 2011, 3314;
- 17bH. Kunkely, V. Pawlowski, A. Strasser, A. Vogler, Inorg. Chem. Commun. 2008, 11, 415;
- 17cH. Kunkely, A. Vogler, Inorg. Chem. Commun. 2004, 7, 770;
- 17dD.-Q. Gao, C.-H. Huang, K. Ibrahim, F.-Q. Liu, Solid State Commun. 2002, 121, 145.
- 18CCDC-932725 (1) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.