Characterization of the Key Intermediates of Carbene-Catalyzed Umpolung by NMR Spectroscopy and X-Ray Diffraction: Breslow Intermediates, Homoenolates, and Azolium Enolates†
Corresponding Author
Prof. Dr. Albrecht Berkessel
Cologne University, Department of Chemistry, Greinstrasse 4, 50939 Cologne (Germany) http://www.berkessel.de
Cologne University, Department of Chemistry, Greinstrasse 4, 50939 Cologne (Germany) http://www.berkessel.deSearch for more papers by this authorVeera Reddy Yatham
Cologne University, Department of Chemistry, Greinstrasse 4, 50939 Cologne (Germany) http://www.berkessel.de
Search for more papers by this authorSilvia Elfert
Cologne University, Department of Chemistry, Greinstrasse 4, 50939 Cologne (Germany) http://www.berkessel.de
Search for more papers by this authorDr. Jörg-M. Neudörfl
Cologne University, Department of Chemistry, Greinstrasse 4, 50939 Cologne (Germany) http://www.berkessel.de
Search for more papers by this authorCorresponding Author
Prof. Dr. Albrecht Berkessel
Cologne University, Department of Chemistry, Greinstrasse 4, 50939 Cologne (Germany) http://www.berkessel.de
Cologne University, Department of Chemistry, Greinstrasse 4, 50939 Cologne (Germany) http://www.berkessel.deSearch for more papers by this authorVeera Reddy Yatham
Cologne University, Department of Chemistry, Greinstrasse 4, 50939 Cologne (Germany) http://www.berkessel.de
Search for more papers by this authorSilvia Elfert
Cologne University, Department of Chemistry, Greinstrasse 4, 50939 Cologne (Germany) http://www.berkessel.de
Search for more papers by this authorDr. Jörg-M. Neudörfl
Cologne University, Department of Chemistry, Greinstrasse 4, 50939 Cologne (Germany) http://www.berkessel.de
Search for more papers by this authorSupport by the Fonds der Chemischen Industrie and by BASF SE is gratefully acknowledged. A.B. acknowledges COST membership (CM0905, Organocatalysis).
Graphical Abstract
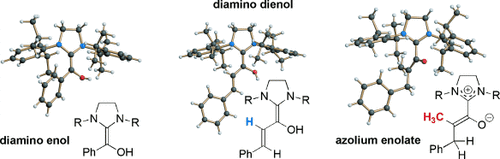
Caught in the act: Diamino enols, diamino dienols, azolium enolates, and azolium enols are postulated intermediates of the N-heterocyclic carbene catalyzed umpolung of aldehydes and enals. Several of these elusive reaction intermediates were generated with the saturated imidazolidin-2-ylidene SIPr (R=2,6-bis(2-propyl)phenyl) and characterized by NMR spectroscopy and X-ray crystallography.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303107_sm_miscellaneous_information.pdf3.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. Breslow, J. Am. Chem. Soc. 1957, 79, 1762–1763.
- 2R. Breslow, J. Am. Chem. Soc. 1958, 80, 3719–3726.
- 3A. Grossmann, D. Enders, Angew. Chem. 2012, 124, 320–332; Angew. Chem. Int. Ed. 2012, 51, 314–325.
- 4D. Enders, O. Niemeier, A. Henseler, Chem. Rev. 2007, 107, 5606–5655.
- 5X. Bugaut, F. Glorius, Chem. Soc. Rev. 2012, 41, 3511–3522.
- 6D. Seebach, Angew. Chem. 1979, 91, 259–278; Angew. Chem. Int. Ed. Engl. 1979, 18, 239–258.
- 7A. Berkessel, S. Elfert, V. R. Yatham, J.-M. Neudörfl, N. E. Schlörer, J. H. Teles, Angew. Chem. 2012, 124, 12537–12541;
10.1002/ange.201205878 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 12370–12374.
- 8J. H. Teles, J.-P. Melder, K. Ebel, R. Schneider, E. Gehrer, W. Harder, S. Brode, D. Enders, K. Breuer, G. Raabe, Helv. Chim. Acta 1996, 79, 61–83.
- 9C. J. Collett, R. S. Massey, O. R. Maguire, A. S. Batsanov, A. C. O’Donoghue, A. D. Smith, Chem. Sci. 2013, 4, 1514–1522.
- 10A. Berkessel, S. Elfert, K. Etzenbach-Effers, J. H. Teles, Angew. Chem. 2010, 122, 7275–7279;
10.1002/ange.200907275 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7120–7124.
- 11
- 11aD. A. DiRocco, K. M. Oberg, T. Rovis, J. Am. Chem. Soc. 2012, 134, 6143–6145;
- 11bD. A. DiRocco, T. Rovis, Angew. Chem. 2012, 124, 6006–6008;
10.1002/ange.201202442 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 5904–5906.
- 12B. Maji, H. Mayr, Angew. Chem. 2012, 124, 10554–10558; Angew. Chem. Int. Ed. 2012, 51, 10408–10412.
- 13Reviews:
- 13aP.-C. Chiang, J. W. Bode, Science of Synthesis: Asymmetric Organocatalysis, Vol. 1 (Ed.: ), Thieme, Stuttgart, 2012, pp. 639–672;
- 13bV. Nair, R. S. Menon, A. T. Biju, C. R. Sinu, R. R. Paul, A. Jose, V. Sreekumar, Chem. Soc. Rev. 2011, 40, 5336–5346.
- 14
- 14aC. Burstein, F. Glorius, Angew. Chem. 2004, 116, 6331–6334;
10.1002/ange.200461572 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 6205–6208;
- 14bS. S. Sohn, E. L. Rosen, J. W. Bode, J. Am. Chem. Soc. 2004, 126, 14370–14371.
- 15Y. Reddi, R. B. Sunoj, Org. Lett. 2012, 14, 2810–2813.
- 16Z. Fu, H. Sun, S. Chen, B. Tiwari, G. Li, Y. R. Chi, Chem. Commun. 2013, 49, 261–263.
- 17J. Kaeobamrung, M. C. Kozlowski, J. W. Bode, Proc. Natl. Acad. Sci. USA 2010, 107, 20661–20665.
- 18S. E. Allen, J. Mahatthananchai, J. W. Bode, M. C. Kozlowski, J. Am. Chem. Soc. 2012, 134, 12098–12103.
- 19
- 19aM. Regitz, J. Hocker, B. Weber, Angew. Chem. 1970, 82, 394–395;
10.1002/ange.19700821010 Google ScholarAngew. Chem. Int. Ed. Engl. 1970, 9, 375–375;
- 19bL. Weber, U. Lassahn, H.-G. Stammler, B. Neumann, Eur. J. Inorg. Chem. 2005, 4590–4597;
- 19cY.-G. Lee, J. P. Moerdyk, C. W. Bielawski, J. Phys. Org. Chem. 2012, 25, 1027–1032;
- 19dM. Hans, J. Wouters, A. Demonceau, L. Delaude, Chem. Eur. J. 2013, 19, 9668–9676.
- 20Upon acceptance of this manuscript, we were notified of a related publication by Mayr and Maji discussing the synthesis and ambident reactivity of azolium enolates derived from NHCs and methyl phenyl ketene: B. Maji, H. Mayr, Angew. Chem. 2013, DOI: ; Angew. Chem. Int. Ed. 2013, DOI: .
- 21I. G. Kaplan, Intermolecular Interactions: Physical Picture, Computational Methods and Model Potentials, Wiley, Hoboken, 2006.
10.1002/047086334X Google Scholar
- 22A. Hoekstra, P. Meertens, A. Vos, Acta Crystallogr. Sect. B 1975, 31, 2813–2817.
- 23C. R. Theocharis, W. Jones, C. N. R. Rao, J. Chem. Soc. Chem. Commun. 1984, 1291–1293.
- 24A non-coplanar arrangement of the diene’s (3 h) double bonds had been proposed for this species before: ref. [25].
- 25S. S. Sohn, J. W. Bode, Org. Lett. 2005, 7, 3873–3876.
- 26J. F. Malone, C. M. Murray, M. H. Charlton, R. Docherty, A. J. Lavery, J. Chem. Soc. Faraday Trans. 1997, 93, 3429–3436.
- 27It is worth noting that in the cases studied, the N-C-N bond angle of the imidazolidine ring is indicative of the imidazolidinylidene (as in the diamino enols 3 a, 3 c, and 3 e and the diamino dienol 3 g) versus the imidazolidinium state (as in the azolium enolates 4 a and 4 b or in the azolium enol 5). In the former case, the N1-C2-N3 angle typically amounts to 108°, whereas the same angle experiences a small but significant widening to 110–111° in the cationic state.
- 28
- 28aB. E. Maki, E. V. Patterson, C. J. Cramer, K. A. Scheidt, Org. Lett. 2009, 11, 3942–3945;
- 28bA. Chan, K. A. Scheidt, Org. Lett. 2005, 7, 905–908.
- 29No reaction was observed within ca. 12 h at room temperature when methanol was added to the diamino dienol 3 g.
- 30J. Mahatthananchai, J. W. Bode, Chem. Sci. 2012, 3, 192–197.
- 31CCDC 911917 (3 a), 911918 (3 c), 911919 (3 e), 911921 (3 g), 911920 (4 a), 911922 (4 b), and 925514 (5) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.