Ligand-Induced Conformational Changes of the Multidrug Resistance Transporter EmrE Probed by Oriented Solid-State NMR Spectroscopy†
This work was supported by NIH grant K22AI083745 and start-up funds from New York University. We thank Prof. Ray Turner and Dr. Denice Bay for helpful discussions, Tracy Stanzel for technical assistance, the National High Magnetic Field Laboratory for preliminary NMR experiments, and Prof. Bobby Arora and Steve Joy for synthesis of BMPS.
Graphical Abstract
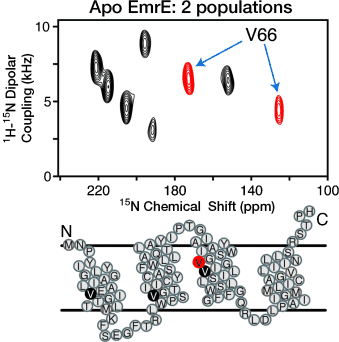
An EmrE-ging market: Oriented solid-state NMR spectroscopy and biochemical cross-linking experiments were used to show that the ligand-free membrane protein transporter EmrE forms anti-parallel dimers with different monomer tilt angles relative to the lipid bilayer. In addition, subtle conformational changes were detected upon drug binding that emphasize the need for an atomic-resolution structure.