[3+2] Fragmentation of an [RP5Cl]+ Cage Cation Induced by an N-Heterocyclic Carbene†
Dr. Michael H. Holthausen
Professur für Anorganische Koordinationschemie, TU Dresden, 01062 Dresden (Germany)
Search for more papers by this authorSabrina K. Surmiak
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany)
Search for more papers by this authorPaul Jerabek
Fachbereich Chemie, Philipps-Universität Marburg, 35032 Marburg (Germany)
Search for more papers by this authorProf. Dr. Gernot Frenking
Fachbereich Chemie, Philipps-Universität Marburg, 35032 Marburg (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jan J. Weigand
Professur für Anorganische Koordinationschemie, TU Dresden, 01062 Dresden (Germany)
Professur für Anorganische Koordinationschemie, TU Dresden, 01062 Dresden (Germany)Search for more papers by this authorDr. Michael H. Holthausen
Professur für Anorganische Koordinationschemie, TU Dresden, 01062 Dresden (Germany)
Search for more papers by this authorSabrina K. Surmiak
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany)
Search for more papers by this authorPaul Jerabek
Fachbereich Chemie, Philipps-Universität Marburg, 35032 Marburg (Germany)
Search for more papers by this authorProf. Dr. Gernot Frenking
Fachbereich Chemie, Philipps-Universität Marburg, 35032 Marburg (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jan J. Weigand
Professur für Anorganische Koordinationschemie, TU Dresden, 01062 Dresden (Germany)
Professur für Anorganische Koordinationschemie, TU Dresden, 01062 Dresden (Germany)Search for more papers by this authorThis work was supported by the Fonds der Chemischen Industrie (FCI, Liebig scholarship for M.H.H.) and the German Science Foundation (DFG, WE 4621/2-1).
Graphical Abstract
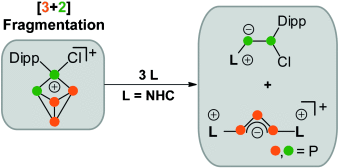
The cage compound [DippP5Cl][GaCl4] (Dipp=2,6-diisopropylphenyl) reacts with an NHC (N-heterocyclic carbene) by an unprecedented [3+2] fragmentation of the P5+ core. This yields an imidazoliumyl-substituted P3 species featuring a triphosphaallyl anion motif and a neutral P2 compound. The mechanism of the fragmentation reaction was elucidated by means of experimental and quantum chemical methods.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201302914_sm_miscellaneous_information.pdf385 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Scheer, G. Balazs, A. Seitz, Chem. Rev. 2010, 110, 4236;
- 1bV. A. Milyukov, Y. H. Budnikova, O. G. Sinyashin, Russ. Chem. Rev. 2005, 74, 781;
- 1cN. A. Giffin, J. D. Masuda, Coord. Chem. Rev. 2011, 255, 1342.
- 2
- 2aD. Holschumacher, T. Bannenberg, K. Ibrom, C. G. Daniliuc, P. G. Jones, M. Tamm, Dalton Trans. 2010, 39, 10590;
- 2bG. Prabusankar, A. Doddi, C. Gemel, M. Winter, R. A. Fischer, Inorg. Chem. 2010, 49, 7976;
- 2cY. Peng, H. Fan, H. Zhu, H. W. Roesky, J. Magull, C. E. Hughes, Angew. Chem. 2004, 116, 3525; Angew. Chem. Int. Ed. 2004, 43, 3443;
- 2dW. Uhl, M. Benter, Chem. Commun. 1999, 771;
- 2eC. Dohmeier, H. Schnöckel, C. Robl, U. Schneider, R. Ahlrichs, Angew. Chem. 1994, 106, 225; Angew. Chem. Int. Ed. Engl. 1994, 33, 199;
- 2fY. Xiong, S. Yao, M. Brym, M. Driess, Angew. Chem. 2007, 119, 4595; Angew. Chem. Int. Ed. 2007, 46, 4511.
- 3T. Köchner, S. Riedel, A. J. Lehner, H. Scherer, I. Raabe, T. A. Engesser, F. W. Scholz, U. Gellrich, P. Eiden, R. A. Paz Schmidt, D. A. Plattner, I. Krossing, Angew. Chem. 2010, 122, 8316;
10.1002/ange.201003031 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 8139.
- 4
- 4aI. Krossing, I. Raabe, Angew. Chem. 2001, 113, 4544;
Angew. Chem. Int. Ed. 2001, 40, 4406;
10.1002/1521-3773(20011203)40:23<4406::AID-ANIE4406>3.0.CO;2-X CAS PubMed Web of Science® Google Scholar
- 4bM. Gonsior, I. Krossing, L. Müller, I. Raabe, M. Jansen, L. van Wüllen, Chem. Eur. J. 2002, 8, 4475;
10.1002/1521-3765(20021004)8:19<4475::AID-CHEM4475>3.0.CO;2-M CAS PubMed Web of Science® Google Scholar
- 4cA. Bihlmeier, M. Gonsior, I. Raabe, N. Trapp, I. Krossing, Chem. Eur. J. 2004, 10, 5041.
- 5
- 5aJ. J. Weigand, M. H. Holthausen, R. Fröhlich, Angew. Chem. 2009, 121, 301; Angew. Chem. Int. Ed. 2009, 48, 295;
- 5bM. H. Holthausen, J. J. Weigand, J. Am. Chem. Soc. 2009, 131, 14210;
- 5cM. H. Holthausen, C. Richter, A. Hepp, J. J. Weigand, Chem. Commun. 2010, 46, 6921;
- 5dM. H. Holthausen, K.-O. Feldmann, S. Schulz, A. Hepp, J. J. Weigand, Inorg. Chem. 2012, 51, 3374;
- 5eM. H. Holthausen, J. J. Weigand, Z. Anorg. Allg. Chem. 2012, 638, 1103.
- 6H. M. Tuononen, R. Roesler, J. L. Dutton, P. J. Ragogna, Inorg. Chem. 2007, 46, 10693.
- 7
- 7aJ. D. Masuda, W. W. Schoeller, B. Donnadieu, G. Bertrand, Angew. Chem. 2007, 119, 7182; Angew. Chem. Int. Ed. 2007, 46, 7052;
- 7bJ. D. Masuda, W. W. Schoeller, B. Donnadieu, G. Bertrand, J. Am. Chem. Soc. 2007, 129, 14180.
- 8O. Back, G. Kuchenbeiser, B. Donnadieu, G. Bertrand, Angew. Chem. 2009, 121, 5638;
10.1002/ange.200902344 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 5530.
- 9See the Supporting Information for further details.
- 10
- 10aE. Niecke, R. Rüger, B. Krebs, Angew. Chem. 1982, 94, 553; Angew. Chem. Int. Ed. Engl. 1982, 21, 544;
- 10bA. R. Fox, R. J. Wright, E. Rivard, P. P. Power, Angew. Chem. 2005, 117, 7907;
10.1002/ange.200502865 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 7729;
- 10cM. Donath, E. Conrad, P. Jerabek, G. Frenking, R. Fröhlich, N. Burford, J. J. Weigand, Angew. Chem. 2012, 124, 3018;
10.1002/ange.201109010 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 2964.
- 11L. Weber, Eur. J. Inorg. Chem. 2000, 2425.
- 12Y. Carpenter, N. Burford, M. D. Lumsden, R. McDonald, Inorg. Chem. 2011, 50, 3342.
- 13
- 13aM. Scheer, C. Kuntz, M. Stubenhofer, M. Linseis, R. F. Winter, M. Sierka, Angew. Chem. 2009, 121, 2638; Angew. Chem. Int. Ed. 2009, 48, 2600;
- 13bM. Scheer, S. Deng, O. J. Scherer, M. Sierka, Angew. Chem. 2005, 117, 3821; Angew. Chem. Int. Ed. 2005, 44, 3755;
- 13cP. Jutzi, U. Meyer, Phosphorus and Sulfur 1988, 40, 275;
- 13dN. Wiberg, A. Wörner, H.-W. Lerner, K. Karaghiosoff, D. Fenske, G. Baum, A. Dransfeld, P. von R. Schleyer, Eur. J. Inorg. Chem. 1998, 833;
10.1002/(SICI)1099-0682(199806)1998:6<833::AID-EJIC833>3.0.CO;2-1 CAS Web of Science® Google Scholar
- 13eV. Thelen, D. Schmidt, M. Nieger, E. Niecke, W. W. Schoeller, Angew. Chem. 1996, 108, 354;
10.1002/ange.19961080322 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 313.
- 14O. Back, B. Donnadieu, P. Parameswaran, G. Frenking, G. Bertrand, Nat. Chem. 2010, 2, 369.
- 15L. Weber, Chem. Rev. 1992, 92, 1839.
- 16V. Cappello, J. Baumgartner, A. Dransfeld, M. Flock, K. Hassler, Eur. J. Inorg. Chem. 2006, 2393.
- 17aH. Hamaguchi, M. Tasumi, M. Yoshifuji, N. Inamoto, J. Am. Chem. Soc. 1984, 106, 508;
- 17bI. A. Garbuzova, L. A. Leites, S. S. Bulkavo, J. Mol. Struct. 1997, 411, 467.
- 18J. J. Weigand, K.-O. Feldmann, F. D. Henne, J. Am. Chem. Soc. 2010, 132, 16321.
- 19rcov(P,P)=2.02 Å, ∑rvdW(P,P)=3.80 Å); A. Bondi, J. Phys. Chem. 1964, 68, 441.
- 20M. P. Mitoraj, A. Michalak, T. Ziegler, J. Chem. Theory Comput. 2009, 5, 962.