Two-Step Protein Self-Assembly in the Extracellular Matrix†
Won Min Park
School of Chemical & Biomolecular Engineering, Georgia Institute of Technology, 311 Ferst Dr. NW, Atlanta, GA 30332 (USA) http://champion.chbe.gatech.edu
Search for more papers by this authorCorresponding Author
Prof. Julie A. Champion
School of Chemical & Biomolecular Engineering, Georgia Institute of Technology, 311 Ferst Dr. NW, Atlanta, GA 30332 (USA) http://champion.chbe.gatech.edu
School of Chemical & Biomolecular Engineering, Georgia Institute of Technology, 311 Ferst Dr. NW, Atlanta, GA 30332 (USA) http://champion.chbe.gatech.eduSearch for more papers by this authorWon Min Park
School of Chemical & Biomolecular Engineering, Georgia Institute of Technology, 311 Ferst Dr. NW, Atlanta, GA 30332 (USA) http://champion.chbe.gatech.edu
Search for more papers by this authorCorresponding Author
Prof. Julie A. Champion
School of Chemical & Biomolecular Engineering, Georgia Institute of Technology, 311 Ferst Dr. NW, Atlanta, GA 30332 (USA) http://champion.chbe.gatech.edu
School of Chemical & Biomolecular Engineering, Georgia Institute of Technology, 311 Ferst Dr. NW, Atlanta, GA 30332 (USA) http://champion.chbe.gatech.eduSearch for more papers by this authorThis research was supported by grants from the National Science Foundation (1032413) and GT Emory Center for Regenerative Engineering & Medicine. We acknowledge J. Park, Prof. A. Bommarius, Dr. I. Mamajanov, and Prof. N. Hud for technical assistance and Profs. K. Zhang and D. Tirrell for plasmid DNA. This work was performed in part at the GT Institute for Electronics and Nanotechnology, a member of the National Nanotechnology Infrastructure Network, which is supported by the NSF.
Graphical Abstract
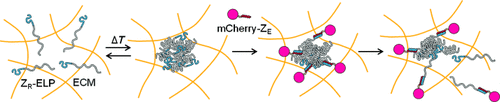
Carrier-free protein delivery: Protein self-assembly can be conducted in the extracellular matrix (ECM) where engineered protein components (ZR-ELP) form particles that become entrapped, bind a model protein (mCherry-ZE), and dissociate. Spontaneous diffusion–coacervation and high-affinity binding of proteins mediate in situ formation of the self-assembled particles that shrink and release the model protein in the ECM (see scheme).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201302331_sm_miscellaneous_information.pdf508.6 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1N. X. Wang, H. A. von Recum, Macromol. Biosci. 2011, 11, 321–332.
- 2B. Amsden, Macromolecules 1998, 31, 8382–8395.
- 3S. E. Sakiyama-Elbert, J. A. Hubbell, J. Controlled Release 2000, 69, 149–158.
- 4K. Vulic, M. S. Shoichet, J. Am. Chem. Soc. 2012, 134, 882–885.
- 5
- 5aB. V. Slaughter, S. S. Khurshid, O. Z. Fisher, A. Khademhosseini, N. A. Peppas, Adv. Mater. 2009, 21, 3307–3329;
- 5bJ. S. Temenoff, H. Shin, D. E. Conway, P. S. Engel, A. G. Mikos, Biomacromolecules 2003, 4, 1605–1613.
- 6T. Kean, M. Thanou, Adv. Drug Delivery Rev. 2010, 62, 3–11.
- 7J. M. Anderson, A. Rodriguez, D. T. Chang, Semin. Immunol. 2008, 20, 86–100.
- 8S. Zhang, Nat. Biotechnol. 2003, 21, 1171–1178.
- 9J. C. Sinclair, K. M. Davies, C. Venien-Bryan, M. E. M. Noble, Nat. Nanotechnol. 2011, 6, 558–562.
- 10
- 10aC. T. S. Wong Po Foo, J. S. Lee, W. Mulyasasmita, A. Parisi-Amon, S. C. Heilshorn, Proc. Natl. Acad. Sci. USA 2009, 106, 22067–22072;
- 10bW. Shen, K. Zhang, J. A. Kornfield, D. A. Tirrell, Nat. Mater. 2006, 5, 153–158;
- 10cA. Bella, S. Ray, M. Shaw, M. G. Ryadnov, Angew. Chem. 2012, 124, 443–446;
10.1002/ange.201104647 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 428–431;
- 10dS. Koutsopoulos, L. D. Unsworth, Y. Nagai, S. Zhang, Proc. Natl. Acad. Sci. USA 2009, 106, 4623;
- 10eS. Toledano, R. J. Williams, V. Jayawarna, R. V. Ulijn, J. Am. Chem. Soc. 2006, 128, 1070–1071.
- 11A. J. Simnick, C. A. Valencia, R. Liu, A. Chilkoti, ACS Nano 2010, 4, 2217–2227.
- 12M. R. Diehl, K. Zhang, H. J. Lee, D. A. Tirrell, Science 2006, 311, 1468–1471.
- 13J. C. T. Carlson, S. S. Jena, M. Flenniken, T.-f. Chou, R. A. Siegel, C. R. Wagner, J. Am. Chem. Soc. 2006, 128, 7630–7638.
- 14O. Lieleg, K. Ribbeck, Trends Cell Biol. 2011, 21, 543–551.
- 15X. Shu, N. C. Shaner, C. A. Yarbrough, R. Y. Tsien, S. J. Remington, Biochemistry 2006, 45, 9639–9647.
- 16K. Zhang, A. Sugawara, D. A. Tirrell, ChemBioChem 2009, 10, 2617–2619.
- 17D. W. Urry, T. L. Trapane, K. U. Prasad, Biopolymers 1985, 24, 2345–2356.
- 18J. R. Moll, S. B. Ruvinov, I. Pastan, C. Vinson, Protein Sci. 2001, 10, 649–655.
- 19T. Stylianopoulos, M.-Z. Poh, N. Insin, M. G. Bawendi, D. Fukumura, L. L. Munn, R. K. Jain, Biophys. J. 2010, 99, 1342–1349.
- 20O. Lieleg, R. M. Baumgärtel, A. R. Bausch, Biophys. J. 2009, 97, 1569–1577.
- 21J. T. Cirulis, C. M. Bellingham, E. C. Davis, D. Hubmacher, D. P. Reinhardt, R. P. Mecham, F. W. Keeley, Biochemistry 2008, 47, 12601–12613.
- 22S. A. Vitale, J. L. Katz, Langmuir 2003, 19, 4105–4110.
- 23
- 23aT. A. T. Lee, A. Cooper, R. P. Apkarian, V. P. Conticello, Adv. Mater. 2000, 12, 1105–1110;
10.1002/1521-4095(200008)12:15<1105::AID-ADMA1105>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 23bM. R. Dreher, A. J. Simnick, K. Fischer, R. J. Smith, A. Patel, M. Schmidt, A. Chilkoti, J. Am. Chem. Soc. 2008, 130, 687–694;
- 23cW. Kim, J. Thévenot, E. Ibarboure, S. Lecommandoux, E. L. Chaikof, Angew. Chem. 2010, 122, 4353–4356; Angew. Chem. Int. Ed. 2010, 49, 4257–4260.
- 24
- 24aJ. A. MacKay, M. Chen, J. R. McDaniel, W. Liu, A. J. Simnick, A. Chilkoti, Nat. Mater. 2009, 8, 993–999;
- 24bD. Y. Furgeson, M. R. Dreher, A. Chilkoti, J. Controlled Release 2006, 110, 362–369.
- 25
- 25aM. F. Shamji, H. Betre, V. B. Kraus, J. Chen, A. Chilkoti, R. Pichika, K. Masuda, L. A. Setton, Arthritis Rheum. 2007, 56, 3650–3661;
- 25bM. F. Shamji, J. Chen, A. H. Friedman, W. J. Richardson, A. Chilkoti, L. A. Setton, J. Controlled Release 2008, 129, 179–186.
- 26D. W. Urry, T. M. Parker, M. C. Reid, D. C. Gowda, J. Bioact. Compat. Polym. 1991, 6, 263–282.
- 27W. Shen, PhD thesis, California Institute of Technology (USA), 2005.
- 28P. Koria, H. Yagi, Y. Kitagawa, Z. Megeed, Y. Nahmias, R. Sheridan, M. L. Yarmush, Proc. Natl. Acad. Sci. USA 2011, 108, 1034–1039.
- 29E. A. Silva, D. J. Mooney, Biomaterials 2010, 31, 1235–1241.