A Base-Stabilized Lead(I) Dimer and an Aromatic Plumbylidenide Anion†
Siew-Peng Chia
Division of Chemistry and Biological Chemistry, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorDr. Hong-Wei Xi
Division of Chemical and Biomolecular Engineering, Nanyang Technological University, 62 Nanyang Drive, Singapore 637459 (Singapore)
Search for more papers by this authorDr. Yongxin Li
Division of Chemistry and Biological Chemistry, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorProf. Dr. Kok Hwa Lim
Division of Chemical and Biomolecular Engineering, Nanyang Technological University, 62 Nanyang Drive, Singapore 637459 (Singapore)
Search for more papers by this authorCorresponding Author
Dr. Cheuk-Wai So
Division of Chemistry and Biological Chemistry, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Division of Chemistry and Biological Chemistry, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)Search for more papers by this authorSiew-Peng Chia
Division of Chemistry and Biological Chemistry, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorDr. Hong-Wei Xi
Division of Chemical and Biomolecular Engineering, Nanyang Technological University, 62 Nanyang Drive, Singapore 637459 (Singapore)
Search for more papers by this authorDr. Yongxin Li
Division of Chemistry and Biological Chemistry, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorProf. Dr. Kok Hwa Lim
Division of Chemical and Biomolecular Engineering, Nanyang Technological University, 62 Nanyang Drive, Singapore 637459 (Singapore)
Search for more papers by this authorCorresponding Author
Dr. Cheuk-Wai So
Division of Chemistry and Biological Chemistry, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Division of Chemistry and Biological Chemistry, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)Search for more papers by this authorThis work is supported by the Central Strategic Initiative for Inter-disciplinary Competitive Fund.
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201301954_sm_miscellaneous_information.pdf406.9 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent reviews, see:
- 1aP. P. Power, Chem. Commun. 2003, 2091;
- 1bP. P. Power, Organometallics 2007, 26, 4362;
- 1cR. C. Fischer, P. P. Power, Chem. Rev. 2010, 110, 3877;
- 1dP. P. Power, Acc. Chem. Res. 2011, 44, 627; For recent articles, see:
- 1eL. Pu, B. Twamley, P. P. Power, J. Am. Chem. Soc. 2000, 122, 3524;
- 1fL. Pu, A. D. Phillips, A. F. Richards, M. Stender, R. S. Simons, M. M. Olmstead, P. P. Power, J. Am. Chem. Soc. 2003, 125, 11626;
- 1gY. Peng, R. C. Fischer, W. A. Merrill, J. Fischer, L. Pu, B. D. Ellis, J. C. Fettinger, R. H. Herber, P. P. Power, Chem. Sci. 2010, 1, 461. See the Supporting Information for other references on heavier Group 14 alkyne analogues. Recently, Jones et al. reported that a digermyne supported by a bulky amido ligand has a singly bonded structure; see Ref. [9d].
- 2N. Takagi, S. Nagase, Organometallics 2007, 26, 469.
- 3
- 3aReview: M. Asay, A. Sekiguchi, Bull. Chem. Soc. Jpn. 2012, 85, 1245;
- 3bJ. S. Han, T. Sasamori, Y. Mizuhata, N. Tokitoh, Dalton Trans. 2010, 39, 9238;
- 3cJ. S. Han, T. Sasamori, Y. Mizuhata, N. Tokitoh, J. Am. Chem. Soc. 2010, 132, 2546.
- 4
- 4aC. Cui, M. M. Olmstead, J. C. Fettinger, G. H. Spikes, P. P. Power, J. Am. Chem. Soc. 2005, 127, 17530;
- 4bR. Kinjo, M. Ichinohe, A. Sekiguchi, J. Am. Chem. Soc. 2007, 129, 26.
- 5Y. Jung, M. Brynda, P. P. Power, M. Head-Gordon, J. Am. Chem. Soc. 2006, 128, 7185.
- 6
- 6aY. Chen, M. Hartmann, M. Diedenhofen, G. Frenking, Angew. Chem. 2001, 113, 2107;
Angew. Chem. Int. Ed. 2001, 40, 2051;
10.1002/1521-3773(20010601)40:11<2051::AID-ANIE2051>3.0.CO;2-D PubMed Web of Science® Google Scholar
- 6bN. Takagi, S. Nagase, Organometallics 2007, 26, 3627.
- 7Only one example was reported: S. Hino, M. M. Olmstead, P. P. Power, Organometallics 2005, 24, 5484.
- 8
- 8aC. Jones, S. J. Bonyhady, N. Holzmann, G. Frenking, A. Stasch, Inorg. Chem. 2011, 50, 12315;
- 8bS. Khan, R. Michel, J. M. Dieterich, R. A. Mata, H. W. Roesky, J.-P. Demers, A. Lange, D. Stalke, J. Am. Chem. Soc. 2011, 133, 17889;
- 8cD. Gau, R. Rodriguez, T. Kato, N. Saffon-Merceron, A. de Cózar, F. P. Cossío, A. Baceiredo, Angew. Chem. 2011, 123, 1124; Angew. Chem. Int. Ed. 2011, 50, 1092;
- 8dS.-P. Chia, R. Ganguly, Y. Li, C.-W. So, Organometallics 2012, 31, 6415;
- 8eS.-P. Chia, H.-X. Yeong, C.-W. So, Inorg. Chem. 2012, 51, 1002;
- 8fS. L. Choong, C. Schenk, A. Stasch, D. Dange, C. Jones, Chem. Commun. 2012, 48, 2504;
- 8gM. Wagner, C. Dietz, S. Krabbe, S. G. Koller, C. Strohmann, K. Jurkschat, Inorg. Chem. 2012, 51, 6851. See the Supporting Information for other references on base-stabilized Group 14 element(I) dimers.
- 9S. S. Sen, D. Kratzert, D. Stern, H. W. Roesky, D. Stalke, Inorg. Chem. 2010, 49, 5786;see the Supporting Information for other references on the reactivities of base-stabilized Group 14 element(I) dimers.
- 10W. J. Hoogervorst, C. J. Elsevier, M. Lutz, A. L. Spek, Organometallics 2001, 20, 4437.
- 11For the details of experimental procedures, see the Supporting Information.
- 12L. Pu, B. Twamley, P. P. Power, Organometallics 2000, 19, 2874.
- 13K. Jurkschat, K. Peveling, M. Schuermann, Eur. J. Inorg. Chem. 2003, 3563.
- 14S. P. Green, C. Jones, A. Stasch, Science 2007, 318, 1754.
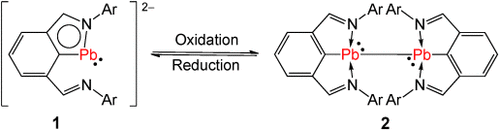
- 15Although [L2Pb:] was not reported, it can be synthesized by the reaction of two equivalents of LLi with PbCl2 in THF. It has been analyzed by X-ray crystallography. For details, see the Supporting Information. With the NMR signals at hand, we can illustrate that [L2Pb:] was formed by the reaction of 2 with one equivalent of Li in THF.
- 16M. Saito, M. Sakaguchi, T. Tajima, K. Ishimura, S. Nagase, M. Hada, Science 2010, 328, 339.
- 17For the details of theoretical studies, see the Supporting Information.
- 18
- 18aP. v. R. Schleyer, M. Manoharan, Z.-X. Wang, B. Kiran, H. Jiao, R. Puchta, N. J. R. v. Eikema Hommes, Org. Lett. 2001, 3, 2465;
- 18bP. v. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao, N. J. R. v. Eikema Hommes, J. Am. Chem. Soc. 1996, 118, 6317.
- 19
- 19aT. Fjeldberg, H. Hope, M. F. Lappert, P. P. Power, A. J. Thorne, J. Chem. Soc. Chem. Commun. 1983, 639;
- 19bP. B. Hitchcock, H. A. Jasim, R. E. Kelly, M. F. Lappert, J. Chem. Soc. Chem. Commun. 1985, 1776;
- 19cA. Jana, H. W. Roesky, C. Schulzke, P. P. Samuel, A. Döring, Inorg. Chem. 2010, 49, 5554.
- 20A. Sekiguchi, T. Fukawa, V. Y. Lee, M. Nakamoto, J. Am. Chem. Soc. 2003, 125, 9250.