Regioselectively Functionalized Pyridines from Sustainable Resources†
This project was supported by the Deutsche Forschungsgemeinschaft (DFG KE 756/23-1).
Graphical Abstract
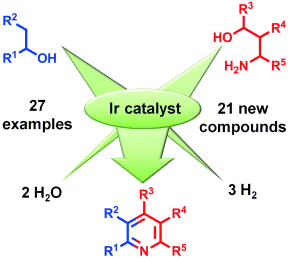
Make the most of it! An Ir-catalyzed dehydrogenative condensation of alcohols and 1,3-amino alcohol was used to construct pyridine derivatives regioselectively. This method provides access to unsymmetrically substituted pyridines and tolerates a wide variety of functional groups. Three equivalents of H2 are generated per pyridine unit formed and the alcohol substrates become completely deoxygenated.