Organocatalytic Synthesis of Highly Functionalized Pyridines at Room Temperature†
Dr. Zugui Shi
Division of Chemistry and Biological Chemistry, Nanyang Technological University, Singapore 637371 (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Teck-Peng Loh
Hefei National Laboratory for Physical Science at the Microscale and Department of Chemistry, University of Sciences and Technology of China, Hefei, Anhui 230026 (P. R. China)
Division of Chemistry and Biological Chemistry, Nanyang Technological University, Singapore 637371 (Singapore)
Hefei National Laboratory for Physical Science at the Microscale and Department of Chemistry, University of Sciences and Technology of China, Hefei, Anhui 230026 (P. R. China)Search for more papers by this authorDr. Zugui Shi
Division of Chemistry and Biological Chemistry, Nanyang Technological University, Singapore 637371 (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Teck-Peng Loh
Hefei National Laboratory for Physical Science at the Microscale and Department of Chemistry, University of Sciences and Technology of China, Hefei, Anhui 230026 (P. R. China)
Division of Chemistry and Biological Chemistry, Nanyang Technological University, Singapore 637371 (Singapore)
Hefei National Laboratory for Physical Science at the Microscale and Department of Chemistry, University of Sciences and Technology of China, Hefei, Anhui 230026 (P. R. China)Search for more papers by this authorWe gratefully acknowledge the Nanyang Technological University, the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2010-T2-2-067, and MOE2011-T2-1-013) for financial support.
Graphical Abstract
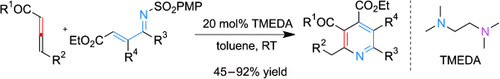
Amines by all means: A unique aza-Rauhut–Currier/cyclization/desulfonation cascade reaction between allenoates and N-sulfonyl-1-aza-1,3-dienes, catalyzed by the readily available diamine TMEDA, has been developed. This strategy provides facile access to a broad range of valuable highly functionalized pyridines in good yields under very mild reaction conditions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201301519_sm_miscellaneous_information.pdf1.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. P. Michael, Nat. Prod. Rep. 2005, 22, 627;
- 1bM. Abass, Heterocycles 2005, 65, 901;
- 1cG. Jones in Comprehensive Heterocyclic Chemistry, Vol. 5 (Eds.: ), Pergamon, Oxford, 1996, p. 167;
10.1016/B978-008096518-5.00108-8 Google Scholar
- 1dJ. A. Joule, K. Mills In heterocyclic Chemistry, 4th ed. Wiley-Blackwell, Oxford, 2000;
- 1eJ. S. Carey, D. Laffan, C. homson, M. T. Williams, Org. Biomol. Chem. 2006, 4, 2337;
- 1fV. C. Gibson, C. Redshaw, G. A. Solan, Chem. Rev. 2007, 107, 1745.
- 2For reviews, see:
- 2aJ. A. Varela, C. Saa, Chem. Rev. 2003, 103, 3787;
- 2bG. D. Henry, Tetrahedron 2004, 60, 6043;
- 2cG. Zeni, R. C. Larock, Chem. Rev. 2006, 106, 4644;
- 2dM. A. Ciufolini, B. K. Chan, Heterocycles 2007, 74, 101;
- 2eB. Heller, M. Hapke, Chem. Soc. Rev. 2007, 36, 1085;
- 2fM. C. Bagley, C. Glover, E. A. Merrit, Synlett 2007, 2459;
- 2gB. Groenendaal, E. Ruijter, R. V. A. Orru, Chem. Commun. 2008, 5474;
- 2hM. D. Hill, Chem. Eur. J. 2010, 16, 12052.
- 3For selected recent achievements on pyridine synthesis, see:
- 3aM. M. McCormick, H. A. Duong, G. Zuo, J. Louie, J. Am. Chem. Soc. 2005, 127, 5030;
- 3bR. Tanaka, A. Yuza, Y. Watai, D. Suzuki, Y. Takayam, F. Sato, H. Urabe, J. Am. Chem. Soc. 2005, 127, 7774;
- 3cM. Movassaghi, M. D. Hill, J. Am. Chem. Soc. 2006, 128, 4592;
- 3dY. Yamamoto, K. Kinpara, R. Ogawa, H. Nishiyama, K. Itoh, Chem. Eur. J. 2006, 12, 5618;
- 3eJ. Dash, T. Lechel, H.-U. Reissig, Org. Lett. 2007, 9, 5541;
- 3fB. M. Trost, A. C. Gutierrez, Org. Lett. 2007, 9, 1473;
- 3gJ. Barluenga, M. A. Fernández-Rodríguez, P. García-García, E. Aguilar, J. Am. Chem. Soc. 2008, 130, 2764;
- 3hJ. R. Manning, H. M. L. Davies, J. Am. Chem. Soc. 2008, 130, 8602;
- 3iS. Liu, L. S. Liebeskind, J. Am. Chem. Soc. 2008, 130, 6918;
- 3jK. Parthasarathy, M. Jeganmohan, C.-H. Cheng, Org. Lett. 2008, 10, 325;
- 3kT. Sasada, N. Sakai, T. Konakahara, J. Org. Chem. 2008, 73, 6905;
- 3lS. Chiba, Y.-J. Xu, Y.-F. Wang, J. Am. Chem. Soc. 2009, 131, 12886;
- 3mY. F. Wang, S. Chiba, J. Am. Chem. Soc. 2009, 131, 12570;
- 3nI. Nakamura, D. Zhang, M. Terada, J. Am. Chem. Soc. 2010, 132, 7884;
- 3oM. Ohashi, I. Takeda, M. Ikawa, S. Ogoshi, J. Am. Chem. Soc. 2011, 133, 18018;
- 3pC. Wang, X. Li, F. Wu, B. Wan, Angew. Chem. 2011, 123, 7300; Angew. Chem. Int. Ed. 2011, 50, 7162;
- 3qS. Yamamoto, K. Okamoto, M. Murakoso, Y. Kuninobu, K. Takai, Org. Lett. 2012, 14, 3182;
- 3rW. Gati, M. M. Rammah, M. B. Rammah, F. Couty, G. Evano, J. Am. Chem. Soc. 2012, 134, 9078.
- 4For reviews on the Rauhut–Currier reaction, see:
- 4aJ. L. Methot, W. R. Roush, Adv. Synth. Catal. 2004, 346, 1035;
- 4bC. E. Aroyan, A. Dermenci, S. J. Miller, Tetrahedron 2009, 65, 4069. For selected examples of intramolecular Rauhut-Currier reaction, see:
- 4cP. M. Brown, N. Káppel, P. J. Murphy, Tetrahedron Lett. 2002, 43, 8707;
- 4dE. Marqués-López, R. P. Herrera, T. Marks, W. C. Jacobs, D. Köning, R. M. Figueiredo, M. Christmann, Org. Lett. 2009, 11, 4116;
- 4eJ. E. Wilson, J. Sun, G. C. Fu, Angew. Chem. 2010, 122, 165;
10.1002/ange.200905125 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 161;
- 4fY. Qiao, S. Kumar, W. P. Malachowski, Tetrahedron Lett. 2010, 51, 2636;
- 4gX.-F. Wang, L. Peng, J. An, C. Li, Q.-Q. Yang, L.-Q. Lu, F.-L. Gu, W.-J. Xiao, Chem. Eur. J. 2011, 17, 6484;
- 4hJ.-J. Gong, T.-Z. Li, K. Pan, X.-Y. Wu, Chem. Commun. 2011, 47, 1491;
- 4iS. Takizawa, T. M. Nguyen, A. Grossmann, D. Enders, H. Sasai, Angew. Chem. 2012, 124, 5519;
10.1002/ange.201201542 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 5423;
- 4jP. S. Selig, S. J. Miller, Tetrahedron Lett. 2011, 52, 2148.
- 5For the intermolecular Rauhut–Currier reaction, see:
- 5aT. E. Reynolds, M. S. Binkley, K. A. Scheidt, Org. Lett. 2008, 10, 2449;
- 5bC. Zhong, Y. Chen, J. L. Petersen, N. G. Akhmedov, X. Shi, Angew. Chem. 2009, 121, 1305; Angew. Chem. Int. Ed. 2009, 48, 1279;
- 5cP. Shanbhag, P. R. Nareddy, M. Dadwal, S. M. Mobin, I. N. N. Namboothiri, Org. Biomol. Chem. 2010, 8, 4867;
- 5dQ.-Y. Zhao, C.-K. Pei, X.-Y. Guan, M. Shi, Adv. Synth. Catal. 2011, 353, 1973;
- 5eZ. Shi, Q. Tong, W. W. Y. Leong, G. Zhong, Chem. Eur. J. 2012, 18, 9802;
- 5fZ. Shi, P. Yu, T.-P. Loh, G. Zhong, Angew. Chem. 2012, 124, 7945; Angew. Chem. Int. Ed. 2012, 51, 7825.
- 6
- 6aC. E. Aroyan, S. J. Miller, J. Am. Chem. Soc. 2007, 129, 256;
- 6bC. E. Aroyan, A. Dermenci, S. J. Miller, J. Org. Chem. 2010, 75, 5784.
- 7For reviews of amine catalysis, see:
- 7aS. France, D. J. Guerin, S. J. Miller, T. Lectka, Chem. Rev. 2003, 103, 2985;
- 7bG. C. Fu, Acc. Chem. Res. 2004, 37, 542;
- 7cR. P. Wurz, Chem. Rev. 2007, 107, 5570;
- 7dS. Tian, Y. Chen, J. Hang, L. Tang, P. McDaid, L. Deng, Acc. Chem. Res. 2004, 37, 621;
- 7eP. I. Dalko, L. Moisan, Angew. Chem. 2004, 116, 5248;
10.1002/ange.200400650 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5138;
- 7fB. List, Acc. Chem. Res. 2004, 37, 548;
- 7gW. Notz, F. Tanaka, C. F. Barbas III, Acc. Chem. Res. 2004, 37, 580;
- 7hM. J. Gaunt, C. C. C. Johansson, Chem. Rev. 2007, 107, 5596;
- 7iD. Enders, C. Grondal, M. R. M. Hüttl, Angew. Chem. 2007, 119, 1590;
10.1002/ange.200603129 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 1570;
- 7jP. Melchiorre, M. Marigo, A. Carlone, G. Bartoli, Angew. Chem. 2008, 120, 6232;
10.1002/ange.200705523 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6138;
- 7kD. W. C. MacMillan, Nature 2008, 455, 304;
- 7lS. E. Denmark, G. L. Beutner, Angew. Chem. 2008, 120, 1584;
10.1002/ange.200604943 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 1560. For selected amine catalyzed reactions, see:
- 7mK. A. Ahrendt, C. J. Borths, D. W. C. MacMillan, J. Am. Chem. Soc. 2000, 122, 4243;
- 7nB. List, R. A. Lerner, C. F. Barbas III, J. Am. Chem. Soc. 2000, 122, 2395;
- 7oG. Zhong, Angew. Chem. 2003, 115, 4379; Angew. Chem. Int. Ed. 2003, 42, 4247;
- 7pJ. Franzén, M. Marigo, D. Fielenbach, T. C. Wabnitz, A. Kjrsgaard, K. A. Jørgensen, J. Am. Chem. Soc. 2005, 127, 18296;
- 7qZ. Tang, Z.-H. Yang, X.-H. Chen, L.-F. Cun, A.-Q. Mi, Y.-Z. Jiang, L.-Z. Gong, J. Am. Chem. Soc. 2005, 127, 9285;
- 7rY. Hayashi, H. Gotoh, T. Hayashi, M. Shoji, Angew. Chem. 2005, 117, 4284; Angew. Chem. Int. Ed. 2005, 44, 4212;
- 7sJ.-W. Xie, W. Chen, R. Li, M. Zeng, W. Du, L. Yue, Y.-C. Chen, Y. Wu, J. Zhu, J.-G. Deng, Angew. Chem. 2007, 119, 393; Angew. Chem. Int. Ed. 2007, 46, 389;
- 7tZ. Shi, P. Yu, P. J. Chua, G. Zhong, Adv. Synth. Catal. 2009, 351, 2797;
- 7uZ. Shi, B. Tan, W. W. Y. Leong, X. Zeng, M. Lu, G. Zhong, Org. Lett. 2010, 12, 5402;
- 7vJ. Xiao, F.-X. Xu, Y.-P. Lu, T.-P. Loh, Org. Lett. 2010, 12, 1220.
- 8For amine-catalyzed cross-Rauhut–Currier reactions, see:
- 8aC. A. Evans, S. J. Miller, J. Am. Chem. Soc. 2003, 125, 12394;
- 8bW. Yao, Y. Wu, G. Wang, Y. Zhang, C. Ma, Angew. Chem. 2009, 121, 9893; Angew. Chem. Int. Ed. 2009, 48, 9713;
- 8cH. Liu, Q. Zhang, L. Wang, X. Tong, Chem. Commun. 2010, 46, 312;
- 8dW. Liu, J. Zhou, C. Zheng, X. Chen, H. Xiao, Y. Yang, Y. Guo, G. Zhao, Tetrahedron 2011, 67, 1768;
- 8eX. Wang, T. Fang, X. Tong, Angew. Chem. 2011, 123, 5473; Angew. Chem. Int. Ed. 2011, 50, 5361.