The Psymberin Story—Biological Properties and Approaches towards Total and Analogue Syntheses†
Corresponding Author
Dr. Max Bielitza
Institut für Bioorganische Chemie der Universität Düsseldorf im Forschungszentrum Jülich, Stetternicher Forst, Geb. 15.8, 52426 Jülich (Germany) http://www.iboc.uni-duesseldorf.de
Institut für Bioorganische Chemie der Universität Düsseldorf im Forschungszentrum Jülich, Stetternicher Forst, Geb. 15.8, 52426 Jülich (Germany) http://www.iboc.uni-duesseldorf.deSearch for more papers by this authorProf. Dr. Jörg Pietruszka
Institut für Bioorganische Chemie der Universität Düsseldorf im Forschungszentrum Jülich, Stetternicher Forst, Geb. 15.8, 52426 Jülich (Germany) http://www.iboc.uni-duesseldorf.de
Search for more papers by this authorCorresponding Author
Dr. Max Bielitza
Institut für Bioorganische Chemie der Universität Düsseldorf im Forschungszentrum Jülich, Stetternicher Forst, Geb. 15.8, 52426 Jülich (Germany) http://www.iboc.uni-duesseldorf.de
Institut für Bioorganische Chemie der Universität Düsseldorf im Forschungszentrum Jülich, Stetternicher Forst, Geb. 15.8, 52426 Jülich (Germany) http://www.iboc.uni-duesseldorf.deSearch for more papers by this authorProf. Dr. Jörg Pietruszka
Institut für Bioorganische Chemie der Universität Düsseldorf im Forschungszentrum Jülich, Stetternicher Forst, Geb. 15.8, 52426 Jülich (Germany) http://www.iboc.uni-duesseldorf.de
Search for more papers by this authorAbbreviations are given in the appendix.
Graphical Abstract
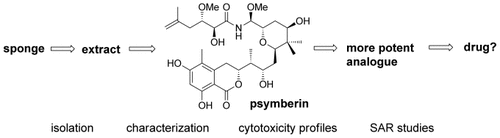
After its isolation from different sponges, the cytotoxic compound psymberin has drawn enormous attention in terms of its structure elucidation and (bio)synthesis because of its fascinating architecture and impressive biological properties. This endeavor resulted in an array of new synthetic strategies that also led to more potent analogues by altering different structural motifs.
Abstract
Psymberin is a marine natural product which has attracted a great deal of interest since its isolation: While the highly cytotoxic compound was detected early on as an ingredient in a marine sponge, it took over a decade and 600 additional samples for the structure to eventually be assigned. In the last eight years fascinating synthetic and biosynthetic investigations have led to a more detailed understanding as well as a new starting point for structure–activity studies towards new antitumor compounds. The Review gives an in-depth insight into the progress in the field of the marine polyketide psymberin and demonstrates how organic synthesis is influencing neighboring scientific subjects.
References
- 1Selected reviews on marine polyketides:
- 1aJ. Staunton, K. J. Weissman, Nat. Prod. Rep. 2001, 18, 380–416;
- 1bW. H. Gerwick, B. S. Moore, Chem. Biol. 2012, 19, 85–98;
- 1cD. A. Akey, J. J. Gehret, D. Khare, J. L. Smith, Nat. Prod. Rep. 2012, 29, 1038–1049, and references therein.
- 2R. H. Cichewicz, F. A. Valeriote, P. Crews, Org. Lett. 2004, 6, 1951–1954.
- 3F. Z. Netolitzky, Z. Angew. Entomol. 1919, 5, 252–257.
10.1111/j.1439-0418.1919.tb01424.x Google Scholar
- 4M. Pavan, G. Bo, Phys. Com. Oecol. 1953, 3, 307–312.
- 5A. Quilico, C. Cardani, D. Chiringhelli, M. Pavan, Chim. Ind. 1961, 43, 1434–1436.
- 6
- 6aC. Cardani, D. Ghiringhelli, R. Mondelli, A. Quilico, Tetrahedron Lett. 1965, 6, 2537–2545;
10.1016/S0040-4039(01)84020-X Google Scholar
- 6bC. Cardani, D. Ghiringhelli, R. Monelli, A. Quilico, Gazz. Chim. Ital. 1966, 96, 3–38.
- 7T. Matsumoto, S. Yanagiya, S. Maeno, S. Yasuda, Tetrahedron Lett. 1968, 9, 6297–6300.
10.1016/S0040-4039(00)75458-X Google Scholar
- 8A. Furusaki, T. Watanabe, T. Matsumoto, M. Yanagiya, Tetrahedron Lett. 1968, 9, 6301–6304.
10.1016/S0040-4039(00)75459-1 Google Scholar
- 9J. H. Frank, K. Kanamitsu, J. Med. Entomol. 1987, 24, 155–191.
- 10A. W. R. McCrae, S. A. Visser, Ann. Trop. Med. Parasitol. 1975, 69, 109–120.
- 11R. K. Armstrong, J. L. Winfield, Am. J. Trop. Med. Hyg. 1969, 18, 147–150.
- 12L. Penchenier, J. Mouchet, B. Cros, P. Legall, J. Y. Cosnefroy, P. Quezede, J. Chandenier, Bull. Soc. Path. Exot. 1994, 87, 45–48.
- 13A. N. Williams, J. R. Army Med. Corps 1993, 139, 17–19.
- 14S. N. R. Qadir, N. Raza, S. B. Rahman, Dermatol. Online J. 2006, 12, 9.
- 15O. Zargari, A. Kimyai-Asadi, F. Fathalikhani, M. Panahi, Int. J. Dermatol. 2003, 42, 608–612.
- 16S. A. Norton, C. Lyons, Lancet 2002, 359, 1950.
- 17Exodus 7:14–12:30.
- 18M. Soldati, A. Fioretti, M. Ghione, Experientia 1966, 22, 176–178.
- 19
- 19aR. A. Mosey, P. E. Floreancig, Nat. Prod. Rep. 2012, 29, 980–995;
- 19bZ. J. Witczak, R. M. Rampulla, A. Bommareddy, Mini-Rev. Med. Chem. 2012, 12, 1520–1532;
- 19cF. Wu, M. E. Green, P. Floreancig, Angew. Chem. 2011, 123, 1163–1166; Angew. Chem. Int. Ed. 2011, 50, 1131–1134 and references therein.
- 20
- 20aJ. Piel, Proc. Natl. Acad. Sci. USA 2002, 99, 14002–14007;
- 20bJ. Piel, I. Höfer, D. Hui, J. Bacteriol. 2004, 186, 1280–1286.
- 21G. R. Pettit, J.-P. Xu, J.-C. Chapuis, R. K. Pettit, L. P. Tackett, D. L. Doubek, J. N. A. Hooper, J. M. Schmidt, J. Med. Chem. 2004, 47, 1149–1152.
- 22S. Kiren, L. Williams, Org. Lett. 2005, 7, 2905–2908.
- 23C. Cardani, C. Fuganti, D. Ghiringhelli, P. Grasselli, M. Pavan, M. D. Valcurone, Tetrahedron Lett. 1973, 14, 2815–2818.
10.1016/S0040-4039(01)96058-7 Google Scholar
- 24K. M. Fisch, C. Gurgui, N. Heycke, S. A. van der Sar, S. A. Anderson, V. L. Webb, S. Taudien, M. Platzer, B. K. Rubio, S. J. Robinson, P. Crews, J. Piel, Nat. Chem. Biol. 2009, 5, 494–501.
- 25A. Schirmer, R. Gadkari, C. D. Reeves, F. Ibrahim, E. F. DeLong, C. R. Hutchinson, Appl. Environ. Microbiol. 2005, 71, 4840–4849.
- 26S. Sudek, N. B. Lopanik, L. E. Waggoner, M. Hildebrand, C. Anderson, H. Liu, A. Patel, D. H. Sherman, M. G. Haygood, J. Nat. Prod. 2007, 70, 67–74.
- 27M. S. Donia, B. J. Hathaway, S. Sudek, M. G. Haygood, M. J. Rosovitz, J. Ravel, E. W. Schmidt, Nat. Chem. Biol. 2006, 2, 729–735.
- 28N. S. Burres, J. J. Clement, Cancer Res. 1989, 49, 2935–2940.
- 29H. Ogawara, K. Higashi, K. Uchino, N. B. Perry, Chem. Pharm. Bull. 1991, 39, 2152–2154.
- 30A. Richter, P. Kocienski, P. Raubo, D. Davies, Anti-Cancer Drug Des. 1997, 12, 217–227.
- 31K. A. Hood, L. M. West, P. T. Northcote, M. V. Berridge, J. H. Miller, Apoptosis 2001, 6, 207–219.
- 32K.-H. Lee, S. Nishimura, S. Matsunaga, N. Fusetani, S. Horinouchi, M. Yoshida, Cancer Sci. 2005, 96, 357–364.
- 33S. Nishimura, S. Matsunaga, M. Yoshida, H. Hirota, S. Yokoyama, N. Fusetani, Bioorg. Med. Chem. 2005, 13, 449–454.
- 34T. Chinen, Y. Nagumo, T. Watanabe, T. Imaizumi, M. Shibuya, T. Kataoka, N. Kanoh, Y. Iwabuchi, T. Usui, Toxicol. Lett. 2010, 199, 341–346.
- 35S. Wan, F. Wu, J. C. Rech, M. E. Green, R. Balachandran, W. S. Horne, B. W. Day, P. E. Floreancig, J. Am. Chem. Soc. 2011, 133, 16668–16679.
- 36C.-Y. Wu, Y. Feng, E. R. Cardenas, N. Williams, P. E. Floreancig, J. K. De Brabander, M. G. Roth, J. Am. Chem. Soc. 2012, 134, 18998–19003.
- 37X. Huang, N. Shao, A. Palani, R. Aslanian, A. Buevich, Org. Lett. 2007, 9, 2597–2600.
- 38G. R. Pettit, C. L. Herald, D. L. Doubek, D. L. Herald, J. Am. Chem. Soc. 1982, 104, 6846–6848.
- 39M. Sasaki, N. Matsumori, T. Maruyama, T. Nonomura, M. Murata, K. Tachibana, T. Yasumoto, Angew. Chem. 1996, 108, 1782–1785; Angew. Chem. Int. Ed. Engl. 1996, 35, 1672–1675.
- 40T. Nonomura, M. Sasaki, N. Matsumori, M. Murata, K. Tachibana, T. Yasumoto, Angew. Chem. 1996, 108, 1786–1789;
10.1002/ange.19961081512 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 1675–1678.
- 41K. Lee, H. Kim, J. Hong, Eur. J. Org. Chem. 2012, 1025–1032.
- 42T. L. B. Boivin, Tetrahedron 1987, 43, 3309–3362.
- 43P. A. Clarke, S. Santos, Eur. J. Org. Chem. 2006, 2045–2053.
- 44I. Larrosa, P. Romea, F. Urpí, Tetrahedron 2008, 64, 2683–2723.
- 45X. Jiang, J. Garcia-Fortanet, J. K. De Brabander, J. Am. Chem. Soc. 2005, 127, 11254–11255.
- 46J. C. Rech, P. E. Floreancig, Org. Lett. 2005, 7, 5175–5178.
- 47N. Shangguan, S. Kiren, L. J. Williams, Org. Lett. 2007, 9, 1093–1096.
- 48A. B. Smith III, J. A. Jurica, S. P. Walsh, Org. Lett. 2008, 10, 5625–5628.
- 49H. Lachance, O. Marion, D. G. Hall, Tetrahedron Lett. 2008, 49, 6061–6064.
- 50K. Gademann, D. E. Chavez, E. N. Jacobsen, Angew. Chem. 2002, 114, 3185–3187;
10.1002/1521-3757(20020816)114:16<3185::AID-ANGE3185>3.0.CO;2-S Google ScholarAngew. Chem. Int. Ed. 2002, 41, 3059–3061.10.1002/1521-3773(20020816)41:16<3059::AID-ANIE3059>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 51L. E. Brown, Y. R. Landaverry, J. R. Davies, K. A. Milinkevich, S. Ast, J. S. Carlson, A. G. Oliver, J. P. Konopelski, J. Org. Chem. 2009, 74, 5405–5410.
- 52M. T. Crimmins, J. M. Stevens, G. M. Schaaf, Org. Lett. 2009, 11, 3990–3993.
- 53T. Watanabe, T. Imaizumi, T. Chinen, Y. Nagumo, M. Shibuya, T. Usui, N. Kanoh, Y. Iwabuchi, Org. Lett. 2010, 12, 1040–1043.
- 54S. R. Byeon, H. Park, H. Kim, J. Hong, Org. Lett. 2011, 13, 5816–5819.
- 55W. J. Buffham, N. A. Swain, S. L. Kostiuk, T. P. Gonçalves, D. C. Harrowven, Eur. J. Org. Chem. 2012, 1217–1222.
- 56M. Bielitza, J. Pietruszka, Synlett 2012, 1625–1628.
- 57Y. Feng, X. Jiang, J. K. De Brabander, J. Am. Chem. Soc. 2012, 134, 17083–17093.
- 58M. Bielitza, J. Pietruszka, Chem. Eur. J. 2013, 19, 8300–8308.
- 59R. D. Barry, Chem. Rev. 1964, 64, 229–260.
- 60N. Choukchou-Braham, Y. Asakawa, J.-P. Lepoittevin, Tetrahedron Lett. 1994, 35, 3949–3952.
- 61J. J. Fitzgerald, A. R. Pagano, V. M. Sakoda, R. A. Olofson, J. Org. Chem. 1994, 59, 4117–4121.
- 62A. Ramacciotti, R. Fiaschi, E. Napolitano, J. Org. Chem. 1996, 61, 5371–5374.
- 63P. Salvadori, S. Superchi, F. Minutolo, J. Org. Chem. 1996, 61, 4190–4191.
- 64S. Superchi, F. Minutolo, D. Pini, P. Salvadori, J. Org. Chem. 1996, 61, 3183–3186.
- 65K. Uchida, H. Watanabe, T. Kitahara, Tetrahedron 1998, 54, 8975–8984.
- 66Y. Kurosaki, T. Fukuda, M. Iwao, Tetrahedron 2005, 61, 3289–3303.
- 67T. Suzuki, T. Yamada, K. Watanabe, T. Katoh, Bioorg. Med. Chem. Lett. 2005, 15, 2583–2585.
- 68S. K. Mandal, S. C. Roy, Tetrahedron Lett. 2007, 48, 4131–4134.
- 69A. Habel, W. Boland, Org. Biomol. Chem. 2008, 6, 1601–1604.
- 70S. K. Mandal, S. C. Roy, Tetrahedron 2008, 64, 11050–11057.
- 71M. Sher, A. Ali, H. Reinke, P. Langer, Tetrahedron Lett. 2008, 49, 5400–5402.
- 72M. D. Obushak, V. S. Matiychuk, V. V. Turytsya, Tetrahedron Lett. 2009, 50, 6112–6115.
- 73A. Rioz-Martínez, G. de Gonzalo, D. E. T. Pazmiño, M. W. Fraaije, V. Gotor, J. Org. Chem. 2010, 75, 2073–2076.
- 74S. Pal, V. Chatare, M. Pal, Curr. Org. Chem. 2011, 15, 782–800.
- 75J. Chen, L. Zhou, C. K. Tan, Y.-Y. Yeung, J. Org. Chem. 2012, 77, 999–1009.
- 76D. Hojo, K. Noguchi, M. Hirano, K. Tanaka, Angew. Chem. 2008, 120, 5904–5906;
10.1002/ange.200801642 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5820–5822.
- 77M. F. Hentemann, J. G. Allen, S. J. Danishefsky, Angew. Chem. 2000, 112, 2013–2016;
10.1002/1521-3757(20000602)112:11<2013::AID-ANGE2013>3.0.CO;2-O Google ScholarAngew. Chem. Int. Ed. 2000, 39, 1937–1940.10.1002/1521-3773(20000602)39:11<1937::AID-ANIE1937>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 78P. Langer, B. Kracke, Tetrahedron Lett. 2000, 41, 4545–4547.
- 79M. E. Green, J. C. Rech, P. E. Floreancig, Org. Lett. 2005, 7, 4117–4120.
- 80B. Henßen, E. Kasparyan, G. Marten, J. Pietruszka, Heterocycles 2007, 245–249.
- 81S. V. Ley, E. Diez, D. J. Dixon, R. T. Guy, P. Michel, G. L. Nattrass, T. D. Sheppard, Org. Biomol. Chem. 2004, 2, 3608–3617.
- 82S. V. Ley, D. J. Dixon, R. T. Guy, M. A. Palomero, A. Polara, F. Rodriguez, T. D. Sheppard, Org. Biomol. Chem. 2004, 2, 3618–3627.
- 83J. Pietruszka, R. C. Simon, Eur. J. Org. Chem. 2009, 3628–3634.
- 84A. B. Northrup, D. W. C. MacMillan, Science 2004, 305, 1752–1755.
- 85A. B. Northrup, I. K. Mangion, F. Hettche, D. W. C. MacMillan, Angew. Chem. 2004, 116, 2204–2206;
10.1002/ange.200453716 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 2152–2154.
- 86D. A. Evans, M. A. Calter, Tetrahedron Lett. 1993, 34, 6871–6874.
- 87X. Huang, N. Shao, A. Palani, R. Aslanian, A. Buevich, C. Seidel-Dugan, R. Huryk, Tetrahedron Lett. 2008, 49, 3592–3595.
- 88S. Wan, M. E. Green, J.-H. Park, P. E. Floreancig, Org. Lett. 2007, 9, 5385–5388.
- 89Q. Xiao, P. E. Floreancig, Org. Lett. 2008, 10, 1139–1142.
- 90M. V. DeBenedetto, M. E. Green, S. Wan, J.-H. Park, P. E. Floreancig, Org. Lett. 2009, 11, 835–838.
- 91C. Lu, Q. Xiao, P. E. Floreancig, Org. Lett. 2010, 12, 5112–5115.
- 92G. Erker, W. Frömberg, J. L. Atwood, W. E. Hunter, Angew. Chem. 1984, 96, 72–73; Angew. Chem. Int. Ed. Engl. 1984, 23, 68–69.
- 93A. Maraval, A. Igau, B. Donnadieu, J.-P. Majoral, Eur. J. Org. Chem. 2003, 385–394.
- 94C. An, A. T. Hoye, A. B. Smith, Org. Lett. 2012, 14, 4350–4353; after acceptance of the present Review a Full Paper was published: C. An, J. A. Jurica, S. P. Walsh, A. T. Hoye, A. B. Smith, J. Org. Chem. 2013, 78, 4278–4296.
- 95X. Jiang, N. Williams, J. K. De Brabander, Org. Lett. 2007, 9, 227–230.
- 96
- 96aX. Huang, N. Shao, R. Huryk, A. Palani, R. Aslanian, C. Seidel-Dugan, Org. Lett. 2009, 11, 867–870;
- 96bN. Shao, X. Huang, A. Palani, R. Aslanian, J. Piwinski, R. Huryk, C. Seidel-Dugan, Synthesis 2009, 2855–2872.
- 97G. R. Pettit, Z. A. Chicacz, F. Gao, C. L. Herald, M. R. Boyd, J. M. Schmidt, J. N. A. Hooper, J. Org. Chem. 1993, 58, 1302–1304.
- 98S. Osman, B. J. Albert, Y. Wang, M. Li, N. L. Czaicki, K. Koide, Chem. Eur. J. 2011, 17, 895–904.