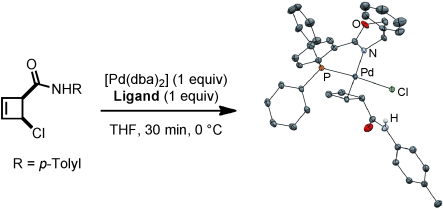Palladium-Catalyzed Allylic Substitution at Four-Membered-Ring Systems: Formation of η1-Allyl Complexes and Electrocyclic Ring Opening†
Dr. Davide Audisio
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany) http://www.kofo.mpg.de/maulide
Search for more papers by this authorDr. Gopinadhanpillai Gopakumar
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany) http://www.kofo.mpg.de/maulide
Search for more papers by this authorDr. Lan-Gui Xie
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany) http://www.kofo.mpg.de/maulide
Search for more papers by this authorDr. Luís G. Alves
Centro de Química Estrutural, Instituto Superior Técnico, Universidade Técnica de Lisboa, Av. Rovisco Pais, 1049-001 Lisbon (Portugal)
Search for more papers by this authorCornelia Wirtz
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany) http://www.kofo.mpg.de/maulide
Search for more papers by this authorProf. Dr. Ana M. Martins
Centro de Química Estrutural, Instituto Superior Técnico, Universidade Técnica de Lisboa, Av. Rovisco Pais, 1049-001 Lisbon (Portugal)
Search for more papers by this authorProf. Dr. Walter Thiel
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany) http://www.kofo.mpg.de/maulide
Search for more papers by this authorDr. Christophe Farès
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany) http://www.kofo.mpg.de/maulide
Search for more papers by this authorCorresponding Author
Dr. Nuno Maulide
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany) http://www.kofo.mpg.de/maulide
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany) http://www.kofo.mpg.de/maulideSearch for more papers by this authorDr. Davide Audisio
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany) http://www.kofo.mpg.de/maulide
Search for more papers by this authorDr. Gopinadhanpillai Gopakumar
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany) http://www.kofo.mpg.de/maulide
Search for more papers by this authorDr. Lan-Gui Xie
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany) http://www.kofo.mpg.de/maulide
Search for more papers by this authorDr. Luís G. Alves
Centro de Química Estrutural, Instituto Superior Técnico, Universidade Técnica de Lisboa, Av. Rovisco Pais, 1049-001 Lisbon (Portugal)
Search for more papers by this authorCornelia Wirtz
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany) http://www.kofo.mpg.de/maulide
Search for more papers by this authorProf. Dr. Ana M. Martins
Centro de Química Estrutural, Instituto Superior Técnico, Universidade Técnica de Lisboa, Av. Rovisco Pais, 1049-001 Lisbon (Portugal)
Search for more papers by this authorProf. Dr. Walter Thiel
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany) http://www.kofo.mpg.de/maulide
Search for more papers by this authorDr. Christophe Farès
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany) http://www.kofo.mpg.de/maulide
Search for more papers by this authorCorresponding Author
Dr. Nuno Maulide
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany) http://www.kofo.mpg.de/maulide
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany) http://www.kofo.mpg.de/maulideSearch for more papers by this authorWe are grateful to the Max-Planck-Society and the Max-Planck-Institut für Kohlenforschung for generous support of our research programs. This work was funded by the ERC (Starting Grant 278872 to N.M.). The invaluable support of our NMR, HPLC, and GC departments as well as Dr. R. Goddard (MPI Mülheim) for crystallographic analysis, is acknowledged. L.G.A. and A.M.M. are grateful to FCT, Portugal (SFRH/BD/44295/2008 and PEst-OE/QUI/UI0100/2011) for financial support.
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201301034_sm_miscellaneous_information.pdf18.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aB. M. Trost, D. L. Van Vranken, Chem. Rev. 1996, 96, 395–422;
- 1bG. Helmchen, U. Kazmaier, S. Förster in Catalytic Asymmetric Synthesis, 3rd ed. ), Wiley, Hoboken, 2010, pp. 497–641;
- 1cG. Poli, G. Prestat, F. Liron, C. Kammerer-Pentier, Top. Organomet. Chem. 2012, 38, 1–63.
- 2
- 2aJ. Sprinz, M. Kiefer, G. Helmchen, G. Huttner, O. Walter, L. Zsolnai, M. Reggelin, Tetrahedron Lett. 1994, 35, 1523–1526;
- 2bS. Y. Liu, J. F. K. Muller, M. Neuburger, S. Schaffner, M. Zehnder, J. Organomet. Chem. 1997, 549, 283–293;
- 2cS. Filipuzzi, P. S. Pregosin, A. Albinati, S. Rizzato, Organometallics 2006, 25, 5955–5964.
- 3Six-membered cyclic π-allyl palladium systems are generally stable and can be fully characterized; for a recent example, see:
- 3aC. P. Butts, E. Filali, G. C. Lloyd-Jones, P.-O. Norrby, D. A. Sale, Y. Schramm, J. Am. Chem. Soc. 2009, 131, 9945–9957; β-hydride elimination has been reported. See:
- 3bB. M. Trost, T. R. Verhoeven, J. M. Fortunak, Tetrahedron Lett. 1979, 20, 2301–2304;
10.1016/S0040-4039(01)93957-7 Google Scholar
- 3cG. R. Cook, P. S. Shanker, Tetrahedron Lett. 1998, 39, 4991–4994.
- 4π-Cyclopentenylpalladium intermediates have been studied by Lloyd-Jones et al., but their isolation and characterization was not possible:
- 4aG. C. Lloyd-Jones, S. C. Stephen, M. Murray, C. P. Butts, Š. Vyskočil, P. Kočovský, Chem. Eur. J. 2000, 6, 4348–4357;
10.1002/1521-3765(20001201)6:23<4348::AID-CHEM4348>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 4bI. J. S. Fairlamb, G. C. Lloyd-Jones, Š. Vyskočil, P. Kočovský, Chem. Eur. J. 2002, 8, 4443–4453.
10.1002/1521-3765(20021004)8:19<4443::AID-CHEM4443>3.0.CO;2-3 CAS PubMed Web of Science® Google Scholar
- 5
- 5aM. Luparia, M. T. Oliveira, D. Audisio, F. Frebault, R. Goddard, N. Maulide, Angew. Chem. 2011, 123, 12840–12844;
10.1002/ange.201106321 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 12631–12635;
- 5bD. Audisio, M. Luparia, M. T. Oliveira, D. Klütt, N. Maulide, Angew. Chem. 2012, 124, 7426–7429;
10.1002/ange.201202853 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 7314–7317.
- 6For detailed 13C chemical shifts in η3-(π)-allyl complexes see:
- 6aJ. M. Rosset, M. P. Glenn, J. D. Cotton, A. C. Willis, C. H. L. Kennard, K. A. Byriel, B. H. Riches, W. Kitching, Organometallics 1998, 17, 1968–1983;
- 6bP. B. Armstrong, L. M. Bennett, R. N. Constantine, J. L. Fields, J. P. Jasinski, R. J. Staples, R. C. Bunt, Tetrahedron Lett. 2005, 46, 1441–1445.
- 7See the Supporting Information for details.
- 8See, e.g.: J. Liu, B. T. Heaton, J. A. Iggo, R. Whyman, Angew. Chem. 2004, 116, 92–96; Angew. Chem. Int. Ed. 2004, 43, 90–94.
- 9
- 9aFor rare examples of η1-allyl Pd complexes with bidentate nonsymmetrical P,N ligands see: M. Kollmar, G. Helmchen, Organometallics 2002, 21, 4771–4775;
- 9bP. Braunstein, Z. A. Jing, R. Welter, Dalton Trans. 2003, 507–509;
- 9cN. H. Sherden, D. C. Behenna, S. C. Virgil, B. M. Stoltz, Angew. Chem. 2009, 121, 6972–6975;
10.1002/ange.200902575 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 6840–6843.
- 10The mechanism accounting for the formation of trans-5 upon reaction of 3 with the malonate nucleophile is intriguing. One might consider coordination of the anion followed by reductive elimination, a pathway that has, to the best of our knowledge, never been observed for allylic alkylations with stabilized (“soft”) nucleophiles. Alternatively, inversion at the metal-bonded carbon should precede nucleophilic attack. Attempts to assess this situation have been unproductive thus far.
- 11The coupling constants of protons Hα (3JHH=14.8 Hz) and Hδ (3JHH=15.2 Hz) were in agreement with this assignment.
- 12
- 12aR. B. Woodward, R. Hoffmann, Angew. Chem. 1969, 81, 797–869;
10.1002/ange.19690812102 Google ScholarAngew. Chem. Int. Ed. Engl. 1969, 8, 781–839;
- 12bE. N. Marwell, Thermal Electrocyclic Reactions, Academic Press, New York, 1980, chap. 5, pp. 124–213.
- 13In the presence of the (2Z,4E)-5-chloropenta-2,4-dienoic acid isomer, intermediate 4 was not observed.
- 14To our knowledge, only five examples of the electrocyclic ring opening of metal/metalloid-substituted cyclobutenes have been reported to date, none of which involve palladium. Silicon:
- 14aM. Murakami, Y. Miyamoto, Y. Ito, Angew. Chem. 2001, 113, 182–184;
Angew. Chem. Int. Ed. 2001, 40, 189–190;
10.1002/1521-3773(20010105)40:1<189::AID-ANIE189>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 14bM. Murakami, Y. Miyamoto, Y. Ito, J. Am. Chem. Soc. 2001, 123, 6441–6442;
- 14cM. Murakami, M. Hasegawa, Angew. Chem. 2004, 116, 4982–4984; Angew. Chem. Int. Ed. 2004, 43, 4874–4876;
- 14dM. Hasegawa, M. Murakami, J. Org. Chem. 2007, 72, 3764–3769. Iron:
- 14eM. C. Chung, X. H. Gu, B. A. Etzenhouser, A. M. Spuches, P. T. Rye, S. K. Seetharaman, D. J. Rose, J. Zubieta, M. B. Sponsler, Organometallics 2003, 22, 3485–3494. Germanium and Tin:
- 14fM. Hasegawa, I. Usui, S. Konno, M. Murakami, Org. Biomol. Chem. 2010, 8, 4169–4175. Zirconium:
- 14gT. Takahashi, Z. Xi, R. Fischer, S. Huo, C. Xi, K. Nakajima, J. Am. Chem. Soc. 1997, 119, 4561–4562.
- 15
- 15aT. I. Son, H. Yanagihara, F. Ozawa, A. Yamamoto, Bull. Chem. Soc. Jpn. 1988, 61, 1251–1258;
- 15bC. Kluwe, J. A. Davies, Organometallics 1995, 14, 4257–4262;
- 15cS. J. Higgins, B. L. Shaw, J. Chem. Soc. Dalton Trans. 1988, 457–460.
- 16When malonate nucleophiles were added to preformed intermediates 7 a and 7 b only decomposition was observed after 15 h at 0 °C. See Ref. [5b].
- 17CCDC 931148 (10) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. See the Supporting Information for more details.
- 18T. V. V. Ramakrishna, S. Lushnikova, P. R. Sharp, Organometallics 2002, 21, 5685–5687.
- 19G. Aullón, D. Bellamy, L. Brammer, E. A. Bruton, A. G. Orpen, Chem. Commun. 1998, 653–654.
- 20See the Supporting Information for a comparative tabular listing of CC bond lengths in several cyclobutene carboxylic acids.