Effect of Multinuclear Copper/Aluminum Complexes in Highly Asymmetric Conjugate Addition of Trimethylaluminum to Acyclic Enones†
Corresponding Author
Prof. Dr. Kohei Endo
Department of Chemistry, Graduate School of Natural Science and Technology, Kanazawa University, Kakuma, Kanazawa, Ishikawa, 920-1192 (Japan)
PRESTO (Japan) Science and Technology Agency (JST), 4-1-8 Honcho Kawaguchi, Saitama, 332-0012 (Japan)
Kohei Endo, Department of Chemistry, Graduate School of Natural Science and Technology, Kanazawa University, Kakuma, Kanazawa, Ishikawa, 920-1192 (Japan)
Takanori Shibata, Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, Ohkubo, Shinjuku, Tokyo 169-8555 (Japan)
Search for more papers by this authorDaisuke Hamada
Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, Ohkubo, Shinjuku, Tokyo 169-8555 (Japan)
Search for more papers by this authorSayuri Yakeishi
Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, Ohkubo, Shinjuku, Tokyo 169-8555 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Takanori Shibata
Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, Ohkubo, Shinjuku, Tokyo 169-8555 (Japan)
Kohei Endo, Department of Chemistry, Graduate School of Natural Science and Technology, Kanazawa University, Kakuma, Kanazawa, Ishikawa, 920-1192 (Japan)
Takanori Shibata, Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, Ohkubo, Shinjuku, Tokyo 169-8555 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Kohei Endo
Department of Chemistry, Graduate School of Natural Science and Technology, Kanazawa University, Kakuma, Kanazawa, Ishikawa, 920-1192 (Japan)
PRESTO (Japan) Science and Technology Agency (JST), 4-1-8 Honcho Kawaguchi, Saitama, 332-0012 (Japan)
Kohei Endo, Department of Chemistry, Graduate School of Natural Science and Technology, Kanazawa University, Kakuma, Kanazawa, Ishikawa, 920-1192 (Japan)
Takanori Shibata, Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, Ohkubo, Shinjuku, Tokyo 169-8555 (Japan)
Search for more papers by this authorDaisuke Hamada
Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, Ohkubo, Shinjuku, Tokyo 169-8555 (Japan)
Search for more papers by this authorSayuri Yakeishi
Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, Ohkubo, Shinjuku, Tokyo 169-8555 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Takanori Shibata
Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, Ohkubo, Shinjuku, Tokyo 169-8555 (Japan)
Kohei Endo, Department of Chemistry, Graduate School of Natural Science and Technology, Kanazawa University, Kakuma, Kanazawa, Ishikawa, 920-1192 (Japan)
Takanori Shibata, Department of Chemistry and Biochemistry, School of Advanced Science and Engineering, Waseda University, Ohkubo, Shinjuku, Tokyo 169-8555 (Japan)
Search for more papers by this authorThis work was supported by the JST PRESTO program, Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science, the Kurata Memorial Hitachi Science and Technology Foundation, and Hokuriku Bank. We thank Ryotaro Takayama (Waseda University) for his experimental assistance.
Graphical Abstract
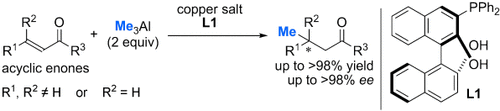
Al and friends: Asymmetric conjugate addition of Me3Al to β,β-disubstituted α,β-unsaturated ketones in the presence copper and L1 leads to a highly efficient construction of an all-carbon-substituted chiral quaternary center. This result is the first example of an asymmetric conjugate addition of Me3Al to acyclic enones to give a chiral quaternary carbon center with excellent yield and enantioselectivity under mild reaction conditions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201206297_sm_miscellaneous_information.pdf22.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aT. Hayashi, K. Yamasaki, Chem. Rev. 2003, 103, 2829;
- 1bA. Alexakis, J. E. Bäckvall, N. Krause, O. Pámies, M. Diéguez, Chem. Rev. 2008, 108, 2796.
- 2Recent examples;
- 2aT. Thaler, P. Knochel, Angew. Chem. 2009, 121, 655;
10.1002/ange.200804446 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 645, and references therein;
- 2bR. Shintani, M. Takeda, T. Nishimura, T. Hayashi, Angew. Chem. 2010, 122, 4061;
10.1002/ange.201000467 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 3969;
- 2cT. L. May, J. A. Dabrowski, A. H. Hoveyda, J. Am. Chem. Soc. 2011, 133, 736;
- 2dK. Kikushima, J. C. Holder, M. Gatti, B. M. Stoltz, J. Am. Chem. Soc. 2011, 133, 6902;
- 2eA. L. Gottumukkala, K. Matcha, M. Lutz, J. G. de Vries, A. J. Minnaard, Chem. Eur. J. 2012, 18, 6907;
- 2fM. Tissot, D. Poggiali, H. Hénon, D. Müller, L. Guénée, M. Mauduit, A. Alexakis, Chem. Eur. J. 2012, 18, 8731.
- 3
- 3aG. Giacomelli, A. M. Caporusso, L. Lardicci, Tetrahedron Lett. 1981, 22, 3663;
- 3bA. M. Caporusso, G. Giacomelli, L. Lardicci, J. Org. Chem. 1982, 47, 4640;
- 3cA. Alberola, C. Andrés, A. G. Ortega, R. Pedrosa, M. Vicente, J. Chem. Soc. Perkin Trans. 1 1987, 2125;
- 3dJ. Kabbara, S. Flemming, K. Nickisch, H. Neh, J. Westermann, Liebigs Ann. 1995, 401;
- 3eP. Magnus, M. J. Waring, C. Ollivier, V. Lynch, Tetrahedron Lett. 2001, 42, 4947;
- 3fY. Matsumoto, K. Yamada, K. Tomioka, J. Org. Chem. 2008, 73, 4578;
- 3gS. Kehrli, D. Martin, D. Rix, M. Mauduit, A. Alexakis, Chem. Eur. J. 2010, 16, 9890.
- 4
- 4aR. Shintani, Y. Tsutsumi, M. Nagaosa, T. Nishimura, T. Hayashi, J. Am. Chem. Soc. 2009, 131, 13588;
- 4bR. Shintani, T. Hayashi, Org. Lett. 2011, 13, 350.
- 5C. Hawner, D. Müller, L. Gremaud, A. Felouat, S. Woodward, A. Alexakis, Angew. Chem. 2010, 122, 7935;
10.1002/ange.201003300 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7769.
- 6For a review:
- 6aA. M. Dumas, E. Fillion, Acc. Chem. Res. 2010, 43, 440;
- 6bA. Wilsily, E. Fillion, J. Org. Chem. 2009, 74, 8583;
- 6cA. Wilsily, T. Lou, E. Fillion, Synthesis 2009, 2066;
- 6dA. Wilsily, E. Fillion, Org. Lett. 2008, 10, 2801;
- 6eE. Fillion, A. Wilsily, J. Am. Chem. Soc. 2006, 128, 2774.
- 7J. Wu, D. M. Mampreian, A. H. Hoveyda, J. Am. Chem. Soc. 2005, 127, 4584.
- 8For a review, see: K. Endo, T. Shibata, Synthesis 2012, 44, 1427.
- 9
- 9aK. Endo, M. Ogawa, T. Shibata, Angew. Chem. 2010, 122, 2460;
10.1002/ange.200906839 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 2410;
- 9bK. Endo, K. Tanaka, M. Ogawa, T. Shibata, Org. Lett. 2011, 13, 868;
- 9cK. Endo, D. Hamada, S. Yakeishi, M. Ogawa, T. Shibata, Org. Lett. 2012, 14, 2342.
- 10The copper-catalyzed asymmetric conjugate addition of organozinc reagents to β-substituted cyclic enones was reported. See:
- 10aA. W. Hird, A. H. Hoveyda, J. Am. Chem. Soc. 2005, 127, 14988;
- 10bK. Lee, M. K. Brown, A. W. Hird, A. H. Hoveyda, J. Am. Chem. Soc. 2006, 128, 7182;
- 10cM. K. Brown, T. L. May, C. A. Baxter, A. H. Hoveyda, Angew. Chem. 2007, 119, 1115; Angew. Chem. Int. Ed. 2007, 46, 1097.
- 11Representative binol/Al complexes:
- 11aT. Arai, Y. M. A. Yamada, N. Yamamoto, H. Sasai, M. Shibasaki, Chem. Eur. J. 1996, 2, 1368;
- 11bT. Arai, H. Sasai, K. Yamaguchi, M. Shibasaki, J. Am. Chem. Soc. 1998, 120, 441;
- 11cE. Emori, T. Iida, M. Shibasaki, J. Org. Chem. 1999, 64, 5318.
- 12P. K. Fraser, S. Woodward, Chem. Eur. J. 2003, 9, 776.
- 13L. Liang, A. S. C. Chan, Tetrahedron: Asymmetry 2002, 13, 1393.
- 14Successful reports of the copper-catalyzed asymmetric conjugate addition of organoaluminum reagents to acyclic enones to give a tertiary stereocenter with more than 80 % ee. See:
- 14aV. Garcia-Ruiz, S. Woodward, Tetrahedron: Asymmetry 2002, 13, 2177;
- 14bA. Alexakis, V. Albrow, K. Biswas, M. d’Augustin, O. Prieto, S. Woodward, Chem. Commun. 2005, 2843;
- 14cN. Fuchs, M. d’Augustin, M. Humam, A. Alexakis, R. Taras, S. Gladiali, Tetrahedron: Asymmetry 2005, 16, 3143;
- 14dY. Mata, M. Diéguez, O. Pámies, K. Biswas, S. Woodward, Tetrahedron: Asymmetry 2007, 18, 1613;
- 14eL. Gremaud, A. Alexakis, Angew. Chem. 2012, 124, 818;
10.1002/ange.201107324 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 794.
- 15See the Supporting information.
- 16M. Vuagnoux-d’Augustin, A. Alexakis, Eur. J. Org. Chem. 2007, 5852.
- 17
- 17aA. Abate, E. Brenna, C. D. Negri, C. Fuganti, S. Serra, Tetrahedron: Asymmetry 2002, 13, 899;
- 17bC. Fuganti, S. Serra, A. Dulio, J. Chem. Soc. Perkin Trans. 1 1999, 279;
- 17cT. P. Loh, Q. Y. Hu, Org. Lett. 2001, 3, 279;
- 17dM. Reiter, S. Torssell, S. Lee, D. W. C. MacMillan, Chem. Sci. 2010, 1, 37.
- 18No conversion using Et2Zn was observed.