Using Host–Guest Complexation to Fold a Flexible Linear Organic String: Kinetically Controlled Syntheses of [3]Catenanes and a Five-Membered Molecular Necklace†
Chia-Fong Chang
Department of Chemistry, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei, Taiwan, 10617 (R.O.C.)
Search for more papers by this authorChun-Ju Chuang
Department of Chemistry, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei, Taiwan, 10617 (R.O.C.)
Search for more papers by this authorProf. Chien-Chen Lai
Institute of Molecular Biology, National Chung Hsing University and Graduate Institute of Chinese Medical Science, China Medical University, Taichung, Taiwan (R.O.C.)
Search for more papers by this authorYi-Hung Liu
Department of Chemistry, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei, Taiwan, 10617 (R.O.C.)
Search for more papers by this authorProf. Shie-Ming Peng
Department of Chemistry, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei, Taiwan, 10617 (R.O.C.)
Search for more papers by this authorCorresponding Author
Prof. Sheng-Hsien Chiu
Department of Chemistry, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei, Taiwan, 10617 (R.O.C.)
Department of Chemistry, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei, Taiwan, 10617 (R.O.C.)Search for more papers by this authorChia-Fong Chang
Department of Chemistry, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei, Taiwan, 10617 (R.O.C.)
Search for more papers by this authorChun-Ju Chuang
Department of Chemistry, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei, Taiwan, 10617 (R.O.C.)
Search for more papers by this authorProf. Chien-Chen Lai
Institute of Molecular Biology, National Chung Hsing University and Graduate Institute of Chinese Medical Science, China Medical University, Taichung, Taiwan (R.O.C.)
Search for more papers by this authorYi-Hung Liu
Department of Chemistry, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei, Taiwan, 10617 (R.O.C.)
Search for more papers by this authorProf. Shie-Ming Peng
Department of Chemistry, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei, Taiwan, 10617 (R.O.C.)
Search for more papers by this authorCorresponding Author
Prof. Sheng-Hsien Chiu
Department of Chemistry, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei, Taiwan, 10617 (R.O.C.)
Department of Chemistry, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei, Taiwan, 10617 (R.O.C.)Search for more papers by this authorWe thank the National Science Council (Taiwan) (NSC-100-2119-M-002-026 and NSC-101-2628-M-002-010) and National Taiwan University (NTU-101-R890913) for financial support.
Graphical Abstract
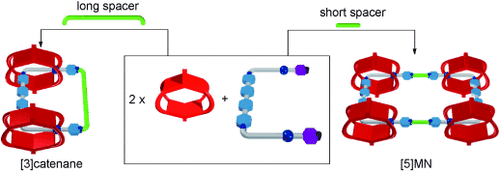
Rings and necklaces: Three [3]catenanes and a five-membered molecular necklace ([5]MN), with up to 60- and 92-membered rings as their centerpieces, respectively, have been synthesized. The synthesis started from the corresponding complexes in which the threaded flexible linear guests were bent at approximately right angles to facilitate kinetically controlled macrocyclizations.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201205498_sm_miscellaneous_information.pdf6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aP. H. Kwan, M. J. MacLachlan, T. M. Swager, J. Am. Chem. Soc. 2004, 126, 8638–8639;
- 1bN.-C. Chen, P.-Y. Huang, C.-C. Lai, Y.-H. Liu, Y. Wang, S.-M. Peng, S.-H. Chiu, Chem. Commun. 2007, 4122–4124;
- 1cO. Hayashida, M. Uchiyama, Org. Biomol. Chem. 2008, 6, 3166–3170;
- 1dJ. J. Gassensmith, S. Matthys, J.-J. Lee, A. Wojcik, P. V. Kamat, B. D. Smith, Chem. Eur. J. 2010, 16, 2916–2921;
- 1eM. J. Chmielewski, J. J. Davis, P. D. Beer, Org. Biomol. Chem. 2009, 7, 415–424;
- 1fD. J. Mercer, S. J. Loeb, Chem. Soc. Rev. 2010, 39, 3612–3620;
- 1gN. H. Evans, C. J. Serpell, P. D. Beer, Chem. Commun. 2011, 47, 8775–8777.
- 2
- 2aJ. W. Lee, K. Kim, Top. Curr. Chem. 2003, 228, 111–140;
- 2bX. Wang, X. Bao, M. McFarland-Mancini, I. Isaacsohn, A. F. Drew, D. B. Smithrud, J. Am. Chem. Soc. 2007, 129, 7284–7293;
- 2cA. Fernandes, A. Viterisi, F. Coutrot, S. Potok, D. A. Leigh, V. Aucagne, S. Papot, Angew. Chem. 2009, 121, 6565–6569;
10.1002/ange.200903215 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 6443–6447;
- 2dM. W. Ambrogio, T. A. Pecorelli, K. Patel, N. M. Khashab, A. Trabolsi, H. A. Khatib, Y. Y. Botros, J. I. Zink, J. F. Stoddart, Org. Lett. 2010, 12, 3304–3307;
- 2eJ. M. Baumes, J. J. Gassensmith, J. Giblin, J.-J. Lee, A. G. White, W. J. Culligan, W. M. Leevy, M. Kuno, B. D. Smith, Nat. Chem. 2010, 2, 1025–1030;
- 2fM. Adeli, R. S. Sarabi, R. Y. Farsi, M. Mahmoudi, M. Kalantari, J. Mater. Chem. 2011, 21, 18686–18695;
- 2gY. Yamada, T. Nomura, H. Harashima, A. Yamashita, N. Yui, Biomaterials 2012, 33, 3952–3958.
- 3
- 3aY.-L. Zhao, I. Aprahamian, A. Trabolsi, N. Erina, J. F. Stoddart, J. Am. Chem. Soc. 2008, 130, 6348–6350;
- 3bS.-Y. Hsueh, C.-T. Kuo, T.-W. Lu, C.-C. Lai, Y.-H. Liu, H.-F. Hsu, S.-M. Peng, C.-h. Chen, S.-H. Chiu, Angew. Chem. 2010, 122, 9356–9359;
10.1002/ange.201004090 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 9170–9173;
- 3cY. Kohsaka, K. Nakazono, Y. Koyama, S. Asai, T. Takata, Angew. Chem. 2011, 123, 4974–4977;
10.1002/ange.201008020 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 4872–4875.
- 4J. Berná, D. A. Leigh, M. Lubomska, S. M. Mendoza, E. M. Pérez, P. Rudolf, G. Teobaldi, F. Zerbetto, Nat. Mater. 2005, 4, 704–710.
- 5
- 5aM. E. Jung, J. Gervay, J. Am. Chem. Soc. 1991, 113, 224–232;
- 5bJ. B. Sperry, D. L. Wright, J. Am. Chem. Soc. 2005, 127, 8034–8035.
- 6
- 6aC. Galli, G. Giovannelli, G. Illuminati, L. Mandolini, J. Org. Chem. 1979, 44, 1258–1261;
- 6bG. Illuminati, L. Mandolini, Acc. Chem. Res. 1981, 14, 95–102.
- 7E. Weber, F. Vogtle, Top. Curr. Chem. 1992, 161, 1–36.
10.1007/3-540-54348-1_6 Google Scholar
- 8
- 8aB. A. Mayes, L. Simon, D. J. Watkin, C. W. G. Ansell, G. W. J. Fleet, Tetrahedron Lett. 2004, 45, 157–162;
- 8bM. Gupta, S. Kang, M. F. Mayer, Tetrahedron Lett. 2008, 49, 2946–2950;
- 8cA. I. Prikhod’ko, J.-P. Sauvage, J. Am. Chem. Soc. 2009, 131, 6794–6807;
- 8dY. Liu, J. Rawlston, A. T. Swann, T. Takatani, C. D. Sherrill, P. J. Ludovice, M. Weck, Chem. Sci. 2011, 2, 429–438.
- 9
- 9aG. W. Gokel, Crown Ethers and Cryptands, Royal Society of Chemistry, London, 1992;
- 9bJ. W. Steed, J. L. Atwood, Supramolecular Chemistry, 2nd ed., Wiley, Chichester, 2009.
10.1002/9780470740880 Google Scholar
- 10Using 1,5-dihydroxynaphthalene and ferrocene derivatives as templates allows cyclobis(paraquat-p-phenylene) and cyclobis(paraquat-4,4′-biphenylene), 28- and 36-membered ring macrocycles, respectively, to be synthesized from their corresponding linear pyridinium salts; see:
- 10aC. L. Brown, D. Philip, J. F. Stoddart, Synlett 1991, 462–464;
- 10bP. R. Ashton, S. Menzer, F. M. Raymo, G. K. H. Shimizu, J. F. Stoddart, D. J. Williams, Chem. Commun. 1996, 487–490.
- 11For reviews, see:
- 11aS. J. Loeb, Chem. Soc. Rev. 2007, 36, 226–235;
- 11bM. D. Pluth, R. G. Bergman, K. N. Raymond, Acc. Chem. Res. 2009, 42, 1650–1659;
- 11cD. J. Tranchemontagne, J. L. Mendoza-Cortes, M. O’Keeffe, O. M. Yaghi, Chem. Soc. Rev. 2009, 38, 1257–1283;
- 11dT. Hügle, M. Hartl, D. Lentz, Chem. Eur. J. 2011, 17, 10184–10207;
- 11eR. Chakrabarty, P. S. Mukherjee, P. J. Stang, Chem. Rev. 2011, 111, 6810–6918.
- 12
- 12aS. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders, J. F. Stoddart, Angew. Chem. 2002, 114, 896–952;
10.1002/1521-3757(20020503)114:9<1528::AID-ANGE11111528>3.0.CO;2-Z Google ScholarAngew. Chem. Int. Ed. 2002, 41, 899–952;10.1002/1521-3773(20020503)41:9<1460::AID-ANIE11111460>3.0.CO;2-N CAS Web of Science® Google Scholar
- 12bP. T. Corbett, J. Leclaire, L. Vial, K. R. West, J.-L. Wietor, J. K. M. Sanders, S. Otto, Chem. Rev. 2006, 106, 3652–3711;
- 12cS. R. Beeren, M. Pittelkow, J. K. M. Sanders, Chem. Commun. 2011, 47, 7359–7361.
- 13
- 13aC.-F. Lin, C.-C. Lai, Y.-H. Liu, S.-M. Peng, S.-H. Chiu, Chem. Eur. J. 2007, 13, 4350–4355;
- 13bC.-J. Chuang, W.-S. Li, C.-C. Lai, Y.-H. Liu, S.-M. Peng, I. Chao, S.-H. Chiu, Org. Lett. 2009, 11, 385–388.
- 14
- 14aN.-C. Chen, C.-C. Lai, Y.-H. Liu, S.-M. Peng, S.-H. Chiu, Chem. Eur. J. 2008, 14, 2904–2908;
- 14bY.-L. Huang, C.-F. Lin, P.-N. Cheng, C.-C. Lai, Y.-H. Liu, S.-M. Peng, S.-H. Chiu, Tetrahedron Lett. 2008, 49, 1665–1669.
- 15K. E. Krakowiak, G. Yi, J. S. Bradshaw, J. Heterocycl. Chem. 1996, 33, 2013–2017.
- 16For a description of the single-point method, see:
- 16aP. R. Ashton, E. J. T. Chrystal, P. T. Glink, S. Menzer, C. Schiavo, N. Spencer, J. F. Stoddart, P. A. Tasker, A. J. P. White, D. J. Williams, Chem. Eur. J. 1996, 2, 709–728;
- 16bP. R. Ashton, M. C. T. Fyfe, S. K. Hickingbottom, J. F. Stoddart, A. J. P. White, D. J. Williams, J. Chem. Soc. Perkin Trans. 2 1998, 2117–2124.
- 17CCDC 889574 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 18Crystal data for [4-H]3PF6: [C110H152O16N3]3PF6⋅4CH3CN; Mr=2377.39; monoclinic; space group C2/c; a=57.243(3); b=13.7391(4); c=36.6570(11) Å; V=26459.6(19) Å3; ρcalcd=1.194 g cm−3; μ(CuKα)=1.134 mm−1; T=200(2) K; yellow prism; 23 750 independent measured reflections; F2 refinement; R1=0.1191; wR2=0.3287.
- 19Molecular necklaces have been defined as cyclic oligorotaxanes comprising a few small rings threaded onto a large macrocycle; see:
- 19aS.-G. Roh, K.-M. Park, G.-J. Park, S. Sakamoto, K. Yamaguchi, K. Kim, Angew. Chem. 1999, 111, 671–675;
Angew. Chem. Int. Ed. 1999, 38, 637–641;
10.1002/(SICI)1521-3773(19990301)38:5<637::AID-ANIE637>3.0.CO;2-4 PubMed Web of Science® Google Scholar
- 19bK. Kim, Chem. Soc. Rev. 2002, 31, 96–107;
- 19cS.-H. Chiu, S. J. Rowan, S. J. Cantrill, L. Ridvan, P. R. Ashton, R. L. Garrell, J. F. Stoddart, Tetrahedron 2002, 58, 807–814;
- 19dS. Dasgupta, J. Wu, Org. Biomol. Chem. 2011, 9, 3504–3515. Molecular necklaces and catenanes are topological isomers. A few high-order catenanes have been synthesized using the “clipping” approach; see:
- 19eD. B. Amabilino, P. R. Ashton, S. E. Boyd, J. Y. Lee, S. Menzer, J. F. Stoddart, D. J. Williams, Angew. Chem. 1997, 109, 2160–2162;
10.1002/ange.19971091909 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 2070–2072;
- 19fD. B. Amabilino, P. R. Ashton, V. Balzani, S. E. Boyd, A. Credi, J. Y. Lee, S. Menzer, J. F. Stoddart, M. Venturi, D. J. Williams, J. Am. Chem. Soc. 1998, 120, 4295–4307;
- 19gL. Fang, M. A. Olson, D. Benitez, E. Tkatchouk, W. A. Goddard III, J. F. Stoddart, Chem. Soc. Rev. 2010, 39, 17–29.
- 20A. Conejo-García, L. Pisani, M. C. Núñez, M. Catto, O. Nicolotti, F. Leonetti, J. M. Campos, M. A. Gallo, A. Espinosa, A. Carotti, J. Med. Chem. 2011, 54, 2627–2645.
- 21The reaction proceeded much slower in the absence of AgPF6. Addition of this salt did not improve the reaction efficiency, however, in the synthesis of the [3]catenane [7-H2]6PF6.