Facilitated Substrate Channeling in a Self-Assembled Trifunctional Enzyme Complex†
This work was supported partially by the DOE BioEnergy Science Center (BESC) and DOE ARPA-E Petro project. This work was also partially supported by the College of Agriculture and Life Sciences Bioprocessing and Biodesign Research Center at Virginia Tech.
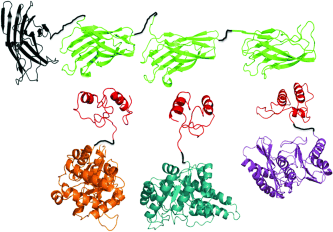
Graphical Abstract
Three enzymes, triosephosphate isomerase (orange in picture), aldolase (cyan), and fructose 1,6-bisphosphatase (purple), which contained dockerins (red), self-assembled into a static trifunctional enzyme complex through interaction with a mini-scaffoldin protein consisting of three different cohesins (green). The synthetic enzyme complex exhibited an enhanced reaction rate compared to the noncomplexed three-enzyme mixture at the same enzyme concentration.