High-Nuclearity Silver Ethynide Clusters Assembled with Phosphonate and Metavanadate Precursors†
Dr. Yun-Peng Xie
Department of Chemistry and Center of Novel Functional Molecules, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong SAR (P.R. China)
Search for more papers by this authorCorresponding Author
Prof. Thomas C. W. Mak
Department of Chemistry and Center of Novel Functional Molecules, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong SAR (P.R. China)
Department of Chemistry and Center of Novel Functional Molecules, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong SAR (P.R. China)Search for more papers by this authorDr. Yun-Peng Xie
Department of Chemistry and Center of Novel Functional Molecules, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong SAR (P.R. China)
Search for more papers by this authorCorresponding Author
Prof. Thomas C. W. Mak
Department of Chemistry and Center of Novel Functional Molecules, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong SAR (P.R. China)
Department of Chemistry and Center of Novel Functional Molecules, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong SAR (P.R. China)Search for more papers by this authorThis work was supported by the Hong Kong Research Grants Council (GRF Ref. CUHK 402710) and the Wei Lun Foundation. We thank The Chinese University of Hong Kong for the award of a Postdoctoral Research Fellowship to Y.-P.X.
Graphical Abstract
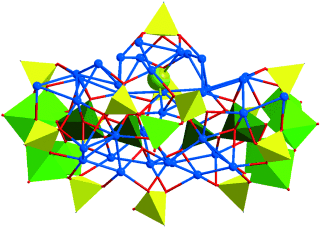
Giant mixed-metal clusters have been assembled with the multinuclear silver(I) tert-butylethynide supramolecular synthon and phosphonate-functionalized oxovanadate building blocks as surface components. Various anionic species can be used as their encapsulated templates. (Picture: Ag36 cluster anion encapsulating a chloride (sphere) and two [(O2)(V2O6)]4− template anions (dark green); Ag blue, O red, P yellow, V green).
References
- 1
- 1a Self-Assembling Architecture (Ed.: ), Alan R. Liss, New York, 1988;
- 1bJ. S. Lindsey, New J. Chem. 1991, 15, 153–180;
- 1cG. M. Whitesides, B. Grzybowski, Science 2002, 295, 2418–2421;
- 1dQ.-F. Sun, J. Iwasa, D. Ogawa, Y. Ishido, S. Sato, T. Ozeki, Y. Sei, K. Yamaguchi, M. Fujita, Science 2010, 328, 1144–1147;
- 1eR. Chakrabarty, P. S. Mukherjee, P. J. Stang, Chem. Rev. 2011, 111, 6810–6918;
- 1fB. H. Northrop, Y.-R. Zheng, K.-W. Chi, P. J. Stang, Acc. Chem. Res. 2009, 42, 1554–1563;
- 1gS. R. Seidel, P. J. Stang, Acc. Chem. Res. 2002, 35, 972–983;
- 1hJ. Ling, J. Qiu, G. E. Sigmon, M. Ward, J. E. S. Szymanowski, P. C. Burns, J. Am. Chem. Soc. 2010, 132, 13395–13402;
- 1iS. T. Zheng, J. Zhang, X. X. Li, W. H. Fang, G. Y. Yang, J. Am. Chem. Soc. 2010, 132, 15102–15103.
- 2
- 2a Polyoxometalate Chemistry: From Topology via Self-Assembly to Applications (Eds.: ), Kluwer, Dordrecht, 2001;
- 2bC. L. Hill, Chem. Rev. 1998, 98, 1–2;
- 2cA. Müller, F. Peters, M. Pope, D. Gatteschi, Chem. Rev. 1998, 98, 239–271;
- 2dN. Mizuno, K. Yamaguchi, K. Kamata, Coord. Chem. Rev. 2005, 249, 1944–1956;
- 2eA. Müller, P. Kögerler, A. W. M. Dress, Coord. Chem. Rev. 2001, 222, 193–218;
- 2fD.-L. Long, E. Burkholder, L. Cronin, Chem. Soc. Rev. 2007, 36, 105–121.
- 3
- 3aP. Putaj, F. Lefebvre, Coord. Chem. Rev. 2011, 255, 1642–1685;
- 3bA. Dolbecq, E. Dumas, C. R. Mayer, P. Mialane, Chem. Rev. 2010, 110, 6009–6048;
- 3c Polyoxometalate Chemistry for Nano-Composite Design (Eds.: ), Kluwer, Dordrecht, 2002.
- 4
- 4aM. I. Khan, J. Zubieta, Angew. Chem. 1994, 106, 784–786; Angew. Chem. Int. Ed. Engl. 1994, 33, 760–762;
- 4bJ. Salta, Q. Chen, Y.-D. Chang, J. Zubieta, Angew. Chem. 1994, 106, 781–783; Angew. Chem. Int. Ed. Engl. 1994, 33, 757–760;
- 4cA. Müller, K. Hovemeier, E. Krickemeyer, H. Bögge, Angew. Chem. 1995, 107, 857–859; Angew. Chem. Int. Ed. Engl. 1995, 34, 779–781;
- 4dS. Konar, A. Clearfield, Inorg. Chem. 2008, 47, 3492–3494;
- 4eS. Khanra, M. Kloth, H. Mansaray, C. A. Muryn, F. Tuna, E. C. San?udo, M. Helliwell, E. J. L. McInnes, R. E. P. Winpenny, Angew. Chem. 2007, 119, 5664–5667;
10.1002/ange.200701115 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 5568–5571;
- 4fA. Müller, R. Rohlfing, J. Döring, M. Penk, Angew. Chem. 1991, 103, 575–577; Angew. Chem. Int. Ed. Engl. 1991, 30, 588–590;
- 4gL. Chen, F. L. Jiang, Z. Z. Lin, Y. F. Zhou, C. Y. Yue, M. C. Hong, J. Am. Chem. Soc. 2005, 127, 8588–8589;
- 4hM.-M. Rohmer, J. Devémy, R. Wiest, M. Bénard, J. Am. Chem. Soc. 1996, 118, 13007–13014;
- 4iM.-M. Rohmer, M. Bénard, J.-P. Blaudeau, J.-M. Maestre, J.-M. Poblet, Coord. Chem. Rev. 1998, 178–180, 1019–1049.
- 5T. C. W. Mak, L. Zhao, Chem. Asian J. 2007, 2, 456–467, and references cited therein. The term “silver ethynide” is preferred to “silver ethynyl” because the silver–carbon bonding interaction is considered to be mainly ionic with minor covalent and components; the negative charge residing mainly on the terminal C atom draws neighboring AgI atoms close to one another to facilitate the onset of argentophilic Ag⋅⋅⋅Ag interactions.
- 6
- 6aS.-D. Bian, H.-B. Wu, Q.-M. Wang, Angew. Chem. 2009, 121, 5467–5469; Angew. Chem. Int. Ed. 2009, 48, 5363–5365;
- 6bS.-D. Bian, J.-H. Jia, Q.-M. Wang, J. Am. Chem. Soc. 2009, 131, 3422–3423;
- 6cJ. Qiao, K. Shi, Q.-M. Wang, Angew. Chem. 2010, 122, 1809–1811; Angew. Chem. Int. Ed. 2010, 49, 1765–1767;
- 6dG. G. Gao, P.-S. Cheng, T. C. W. Mak, J. Am. Chem. Soc. 2009, 131, 18257–18259;
- 6eF. Gruber, M. Jansen, Angew. Chem. 2010, 122, 5044–5046; Angew. Chem. Int. Ed. 2010, 49, 4924–4926;
- 6fS. C. K. Hau, P.-S. Cheng, T. C. W. Mak, J. Am. Chem. Soc. 2012, 134, 2922–2925;
- 6gY.-P. Xie, T. C. W. Mak, J. Am. Chem. Soc. 2011, 133, 3760–3763;
- 6hY.-P. Xie, T. C. W. Mak, Chem. Commun. 2012, 48, 1123–1125.
- 7Crystallographic data: 1: orthorhombic, a=26.504(3), b=35.718(4), c=15.074(2) Å, V=14270(3) Å3, T=173 K, space group Pnma, Z=8, λ=0.71073 Å, ρ=2.258 cm−3, μ(MoKα)=3.425 mm−1, R1=0.0588, wR2=0.1137 for I>2σ(I), GOF=1.166. 2: triclinic, a=21.617(4), b=25.383(5), c=30.016(6) Å, α=90.0, β=108.95(3), γ=117.75(3), V=13559(5) Å3, T=173 K, space group P
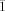 , Z=2, λ=0.71073 Å, ρ=2.046 cm−3, μ(MoKα)=3.058 mm−1, R1=0.1477, wR2=0.2854 for I>2σ(I), GOF=1.103. 3: triclinic, a=23.741(2), b=24.399(2), c=26.387(3) Å, α=96.403(2), β=97.415(2), γ=108.922(2), V=14146(2) Å3, T=173 K, space group P
, Z=2, λ=0.71073 Å, ρ=2.046 cm−3, μ(MoKα)=3.058 mm−1, R1=0.1477, wR2=0.2854 for I>2σ(I), GOF=1.103. 3: triclinic, a=23.741(2), b=24.399(2), c=26.387(3) Å, α=96.403(2), β=97.415(2), γ=108.922(2), V=14146(2) Å3, T=173 K, space group P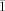 , Z=2, λ=0.71073 Å, ρ=2.052 cm−3, μ(MoKα)=2.984 mm−1, R1=0.0843, wR2=0.2040 for I>2σ(I), GOF=1.057. CCDC 871840 (1), CCDC 871841 (2), and CCDC 871842 (3) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
, Z=2, λ=0.71073 Å, ρ=2.052 cm−3, μ(MoKα)=2.984 mm−1, R1=0.0843, wR2=0.2040 for I>2σ(I), GOF=1.057. CCDC 871840 (1), CCDC 871841 (2), and CCDC 871842 (3) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 8
- 8aD. Rais, J. Yau, D. M. P. Mingos, R. Vilar, A. J. P. White, D. J. Williams, Angew. Chem. 2001, 113, 3572–3575;
10.1002/1521-3757(20010917)113:18<3572::AID-ANGE3572>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 2001, 40, 3464–3467;10.1002/1521-3773(20010917)40:18<3464::AID-ANIE3464>3.0.CO;2-M CAS PubMed Web of Science® Google Scholar
- 8bS.-D. Bian, Q.-M. Wang, Chem. Commun. 2008, 5586–5588.
- 9T. Kurata, A. Uehara, Y. Hayashi, K. Isobe, Inorg. Chem. 2005, 44, 2524–2530.