Highly Regioselective Migration of the Sulfonyl Group: Easy Access to Functionalized Pyrroles†
Xiaoyi Xin
Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian 116023 (China)
Search for more papers by this authorDr. Dongping Wang
Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian 116023 (China)
Search for more papers by this authorDr. Xincheng Li
Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian 116023 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Boshun Wan
Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian 116023 (China)
Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian 116023 (China)Search for more papers by this authorXiaoyi Xin
Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian 116023 (China)
Search for more papers by this authorDr. Dongping Wang
Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian 116023 (China)
Search for more papers by this authorDr. Xincheng Li
Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian 116023 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Boshun Wan
Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian 116023 (China)
Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian 116023 (China)Search for more papers by this authorWe are grateful to the National Natural Science Foundation of China (21172218) and the National Basic Research Program of China (2010CB833300) for financial support.
Graphical Abstract
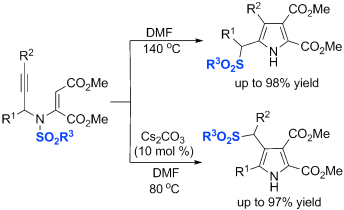
Good migrations: The highly regioselective migration of a sulfonyl group for the synthesis of functionalized pyrroles is reported (see scheme; DMF=N,N-dimethylformamide). The migration of the sulfonyl group to different positions can be controlled with high selectivity, thus allowing the formation of both α- and β-(arylsulfonyl)methyl pyrroles with high yields.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201108144_sm_miscellaneous_information.pdf3.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Pyrroles are one of the most important classes of heterocyclic compounds. See:
- 1aH. N. Fan, J. Peng, M. T. Hamann, J. F. Hu, Chem. Rev. 2008, 108, 264;
- 1bM. d’Ischia, A. Napolitano, A. Pezzella in Comprehensive Heterocyclic Chemistry III, Vol. 3 (Eds.: ), Elsevier, Amsterdam, 2008, pp. 353–388.
10.1016/B978-008044992-0.00304-7 Google Scholar
- 2For reviews on the synthesis of pyrroles, see:
- 2aI. Nakamura, Y. Yamamoto, Chem. Rev. 2004, 104, 2127;
- 2bJ. Bergman, T. Janosik in Comprehensive Heterocyclic Chemistry III, Vol. 3 (Eds.: ), Elsevier, Amsterdam, 2008, pp. 269–351.
10.1016/B978-008044992-0.00303-5 Google Scholar
- 3For recent reports on the synthesis of pyrroles, see:
- 3aK. K. Toh, Y. F. Wang, E. P. J. Ng, S. Chiba, J. Am. Chem. Soc. 2011, 133, 13942;
- 3bB. M. Trost, J. P. Lumb, J. M. Azzarelli, J. Am. Chem. Soc. 2011, 133, 740;
- 3cM. Sai, H. Yorimitsu, K. Oshima, Angew. Chem. 2011, 123, 3352; Angew. Chem. Int. Ed. 2011, 50, 3294;
- 3dM. P. Huestis, L. Chan, D. R. Stuart, K. Fagnou, Angew. Chem. 2011, 123, 1374; Angew. Chem. Int. Ed. 2011, 50, 1338;
- 3eT. Wang, X. L. Chen, L. Chen, Z. P. Zhan, Org. Lett. 2011, 13, 3324;
- 3fH. Y. Wang, D. S. Mueller, R. M. Sachwani, R. Kapadia, H. N. Londino, L. L. Anderson, J. Org. Chem. 2011, 76, 3203;
- 3gD. M. Barber, H. Sanganee, D. J. Dixon, Chem. Commun. 2011, 47, 4379;
- 3hS. Rakshit, F. W. Patureau, F. Glorius, J. Am. Chem. Soc. 2010, 132, 9585;
- 3iD. R. Stuart, P. Alsabeh, M. Kuhn, K. Fagnou, J. Am. Chem. Soc. 2010, 132, 18326;
- 3jM. S. T. Morin, D. J. St-Cyr, B. A. Arndtsen, Org. Lett. 2010, 12, 4916;
- 3kW. B. Liu, H. F. Jiang, L. B. Huang, Org. Lett. 2010, 12, 312;
- 3lA. Mizuno, H. Kusama, N. Iwasawa, Angew. Chem. 2009, 121, 8468;
10.1002/ange.200904402 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 8318;
- 3mN. K. Pahadi, M. Paley, R. Jana, S. R. Waetzig, J. A. Tunge, J. Am. Chem. Soc. 2009, 131, 16626;
- 3nB. Tan, Z. G. Shi, P. J. Chua, Y. X. Li, G. F. Zhong, Angew. Chem. 2009, 121, 772; Angew. Chem. Int. Ed. 2009, 48, 758;
- 3oM. Egi, K. Azechi, S. Akai, Org. Lett. 2009, 11, 5002;
- 3pA. Aponick, C. Y. Li, J. Malinge, E. F. Marques, Org. Lett. 2009, 11, 4624;
- 3qY. D. Lu, B. A. Arndtsen, Org. Lett. 2009, 11, 1369;
- 3rA. R. Kelly, M. H. Kerrigan, P. J. Walsh, J. Am. Chem. Soc. 2008, 130, 4097;
- 3sA. S. Dudnik, A. W. Sromek, M. Rubina, J. T. Kim, A. V. Kel’in, V. Gevorgyan, J. Am. Chem. Soc. 2008, 130, 1440;
- 3tY. D. Lu, B. A. Arndtsen, Angew. Chem. 2008, 120, 5510; Angew. Chem. Int. Ed. 2008, 47, 5430.
- 4Sulfones are widely found in biologically active compounds, for example, the γ-secretase inhibitor for Alzheime’s disease. See:
- 4aJ. Su, H. Q. Tang, B. A. McKittrick, Tetrahedron Lett. 2011, 52, 3382;
- 4bJ. Su, H. Q. Tang, B. A. McKittrick, R. Xu, J. W. Clader, W. J. Greenlee, L. Hyde, L. L. Zhang, Bioorg. Med. Chem. Lett. 2011, 21, 3447.
- 5Sulfones in organic synthesis:
- 5aM. Jegelka, B. Plietker, Chem. Eur. J. 2011, 17, 10417;
- 5bA. R. Alba, X. Companyó, R. Rios, Chem. Soc. Rev. 2010, 39, 2018;
- 5c Sulfones in Organic Synthesis (Ed.: ), Pergamon, Oxford, 1993.
- 6CCDC 808794 (2 a) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 7Proposed gold-catalyzed aza-Claisen rearrangement for pyrrole synthesis. See:
- 7aA. Saito, T. Konishi, Y. Hanzawa, Org. Lett. 2010, 12, 372;
- 7bF. M. Istrate, F. Gagosz, Org. Lett. 2007, 9, 3181.
- 8Utilized Claisen rearrangement intermediate then introduced amines for pyrrole synthesis. See: J. T. Binder, S. F. Kirsch, Org. Lett. 2006, 8, 2151; and for 1, 2-dihydropyridine synthesis, see: T. Harschneck, S. F. Kirsch, J. Org. Chem. 2011, 76, 2145.
- 9E. Benedetti, G. Lemière, L. L. Chapellet, A. Penoni, G. Palmisano, M. Malacria, J. P. Goddard, L. Fensterbank, Org. Lett. 2010, 12, 4396.
- 10CCDC 837830 (3 a) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 11For details of the screening of reaction temperature and reaction time, please see the Supporting Information.