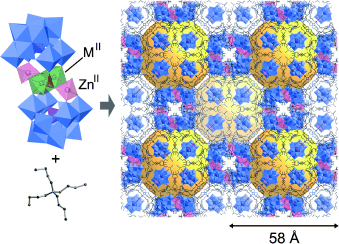
Three-Dimensional Ordered Arrays of 58×58×58 Å3 Hollow Frameworks in Ionic Crystals of M2Zn2-Substituted Polyoxometalates†
Dr. Kosuke Suzuki
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Search for more papers by this authorDr. Yuji Kikukawa
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Search for more papers by this authorDr. Sayaka Uchida
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Search for more papers by this authorDr. Hiroko Tokoro
Department of Chemistry, School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Search for more papers by this authorKenta Imoto
Department of Chemistry, School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Search for more papers by this authorProf. Dr. Shin-ichi Ohkoshi
Department of Chemistry, School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Noritaka Mizuno
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)Search for more papers by this authorDr. Kosuke Suzuki
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Search for more papers by this authorDr. Yuji Kikukawa
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Search for more papers by this authorDr. Sayaka Uchida
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Search for more papers by this authorDr. Hiroko Tokoro
Department of Chemistry, School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Search for more papers by this authorKenta Imoto
Department of Chemistry, School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Search for more papers by this authorProf. Dr. Shin-ichi Ohkoshi
Department of Chemistry, School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Noritaka Mizuno
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)Search for more papers by this authorThis work was supported by the Global COE Program (Chemistry Innovation through Cooperation of Science and Engineering), Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Science, Sports, and Technology of Japan (MEXT), Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program). M=Co, Ni, Zn.
Graphical Abstract
A void in the issue: A one-step self-assembly of γ-Keggin sandwich-type silicotungstates with M2Zn2 (M=Co, Ni, Zn) tetranuclear cores and tetrabutylammonium cations gave porous ionic crystals. These porous crystals are hollow frameworks containing large voids (ca. 38×38×38 Å3, see picture, voids yellow), between which guest molecules can be exchanged through the connecting channels.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201107041_sm_miscellaneous_information.pdf707.6 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. A. van Santen, G. J. Kramer, Chem. Rev. 1995, 95, 637–660;
- 1bA. Corma, Chem. Rev. 1995, 95, 559–614;
- 1cJ. M. Thomas, Angew. Chem. 1999, 111, 3800–3843;
10.1002/(SICI)1521-3757(19991216)111:24<3800::AID-ANGE3800>3.0.CO;2-1 Web of Science® Google ScholarAngew. Chem. Int. Ed. 1999, 38, 3588–3628;10.1002/(SICI)1521-3773(19991216)38:24<3588::AID-ANIE3588>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 1dA. P. Wight, M. E. Davis, Chem. Rev. 2002, 102, 3589–3614.
- 2
- 2aD. J. Tranchemontagne, Z. Ni, M. O’Keeffe, O. M. Yaghi, Angew. Chem. 2008, 120, 5214–5225;
10.1002/ange.200705008 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5136–5147;
- 2bS. Kitagawa, R. Kitaura, S.-i. Noro, Angew. Chem. 2004, 116, 2388–2430; Angew. Chem. Int. Ed. 2004, 43, 2334–2375;
- 2cG. Férey, Chem. Soc. Rev. 2008, 37, 191–214;
- 2dL. J. Murray, M. Dincă, J. R. Long, Chem. Soc. Rev. 2009, 38, 1294–1314;
- 2eY. Inokuma, M. Kawano, M. Fujita, Nat. Chem. 2011, 3, 349–358.
- 3
- 3aH. M. El-Kaderi, J. R. Hunt, J. L. Mendoza-Cortés, A. P. Côté, R. E. Taylor, M. O’Keeffe, O. M. Yaghi, Science 2007, 316, 268–272;
- 3bA. P. Côté, A. I. Benin, N. W. Ockwig, M. O’Keeffe, A. J. Matzger, O. M. Yaghi, Science 2005, 310, 1166–1170.
- 4
- 4aC. L. Hill, C. M. Prosser-McCartha, Coord. Chem. Rev. 1995, 143, 407–455;
- 4bT. Okuhara, N. Mizuno, M. Misono, Adv. Catal. 1996, 41, 113–252;
- 4cR. Neumann, Prog. Inorg. Chem. 1998, 47, 317–370;
- 4dU. Kortz, A. Müller, J. van Slageren, J. Schnack, N. S. Dalal, M. Dressel, Coord. Chem. Rev. 2009, 253, 2315–2327;
- 4eD.-L. Long, R. Tsunashima, L. Cronin, Angew. Chem. 2010, 122, 1780–1803; Angew. Chem. Int. Ed. 2010, 49, 1736–1758;
- 4fA. Müller, E. Krickemeyer, J. Meyer, H. Bögge, F. Peters, W. Plass, E. Diemann, S. Dillinger, F. Nonnenbruch, M. Randerath, C. Menke, Angew. Chem. 1995, 107, 2293–2295; Angew. Chem. Int. Ed. Engl. 1995, 34, 2122–2124;
- 4gH. N. Miras, G. J. T. Cooper, D.-L. Long, H. Bögge, A. Müller, C. Streb, L. Cronin, Science 2010, 327, 72–74;
- 4hK. Kamata, K. Yonehara, Y. Sumida, K. Yamaguchi, S. Hikichi, N. Mizuno, Science 2003, 300, 964–966.
- 5
- 5aM. Hölscher, U. Englert, B. Zibrowius, W. F. Hölderich, Angew. Chem. 1994, 106, 2552–2554; Angew. Chem. Int. Ed. Engl. 1994, 33, 2491–2493;
- 5bM. I. Khan, E. Yohannes, D. Powell, Inorg. Chem. 1999, 38, 212–213;
- 5cJ.-H. Son, H. Choi, Y.-U. Kwon, J. Am. Chem. Soc. 2000, 122, 7432–7433;
- 5dJ.-H. Son, Y.-U. Kwon, Inorg. Chem. 2004, 43, 1929–1932;
- 5eJ.-H. Son, Y.-U. Kwon, Inorg. Chem. 2003, 42, 4153–4159;
- 5fY. Ishii, Y. Takenaka, K. Konishi, Angew. Chem. 2004, 116, 2756–2759; Angew. Chem. Int. Ed. 2004, 43, 2702–2705;
- 5gS. Uchida, M. Hashimoto, N. Mizuno, Angew. Chem. 2002, 114, 2938–2941;
10.1002/1521-3757(20020802)114:15<2938::AID-ANGE2938>3.0.CO;2-P Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2814–2817;10.1002/1521-3773(20020802)41:15<2814::AID-ANIE2814>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 5hS. Uchida, R. Kawamoto, T. Akatsuka, S. Hikichi, N. Mizuno, Chem. Mater. 2005, 17, 1367–1375;
- 5iC. Jiang, A. Lesbani, R. Kawamoto, S. Uchida, N. Mizuno, J. Am. Chem. Soc. 2006, 128, 14240–14241.
- 6
- 6aT. M. Anderson, K. I. Hardcastle, N. Okun, C. L. Hill, Inorg. Chem. 2001, 40, 6418–6425;
- 6bT. M. Anderson, X. Zhang, K. I. Hardcastle, C. L. Hill, Inorg. Chem. 2002, 41, 2477–2488;
- 6cI. M. Mbomekalle, B. Keita, L. Nadjo, W. A. Neiwert, L. Zhang, K. I. Hardcastle, C. L. Hill, T. M. Anderson, Eur. J. Inorg. Chem. 2003, 3924–3928;
- 6dL. Ruhlmann, C. Costa-Coquelard, J. Canny, R. Thouvenot, Eur. J. Inorg. Chem. 2007, 1493–1500;
- 6eD. Schaming, J. Canny, K. Boubekeur, R. Thouvenot, L. Ruhlmann, Eur. J. Inorg. Chem. 2009, 5004–5009;
- 6fC. M. Tourné, G. F. Tourné, F. Zonnevijlle, J. Chem. Soc. Dalton Trans. 1991, 143–155;
- 6gR. Neumann, A. M. Khenkin, Inorg. Chem. 1995, 34, 5753–5760;
- 6hI. M. Mbomekalle, B. Keita, M. Nierlich, U. Kortz, P. Berthet, L. Nadjo, Inorg. Chem. 2003, 42, 5143–5152;
- 6iR. Cao, K. P. O’Halloran, D. A. Hillesheim, S. Lense, K. I. Hardcastle, C. L. Hill, CrystEngComm 2011, 13, 738–740.
- 7Y. Kikukawa, K. Yamaguchi, N. Mizuno, Angew. Chem. 2010, 122, 6232–6236;
10.1002/ange.201001468 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 6096–6100.
- 8The magnetic data of 1 were analyzed by using the analytic expression for a magnetically anisotropic distorted octahedral CoII dimer, and the following best fitting parameters were obtained: J=5.37 cm−1, gz=2.72, gx=4.90, χTIP=190×10−6 cm3 mol−1, D=181.6 cm−1:
- 8aM. E. Lines, J. Chem. Phys. 1971, 55, 2977–2984;
- 8bH. Sakiyama, Inorg. Chim. Acta 2006, 359, 2097–2100.
- 9The magnetic data of 2 were analyzed by using the analytic expression for an octahedral NiII dimer assuming the isotropic spin-coupling Hamiltonian and considering zero-field splitting D. The following best fitting parameters were obtained: J=10.8 cm−1, g=2.01, χTIP=430×10−6 cm3 mol−1, D=6.9 cm−1, where J is exchange interaction parameter, gx and gz are the anisotropic g-factors, χTIP is temperature independent paramagnetism, and D is zero-filed splitting: A. P. Ginsberg, R. L. Martin, R. W. Brookes, R. C. Sherwood, Inorg. Chem. 1972, 11, 2884–2889.
- 1090–100° angles of CoII-O-CoII and NiII-O-NiII favor the orthogonality of the magnetic orbital and result in the ferromagnetic coupling as expected from the Goodenough–Kanamori rule: J. B. Goodenough, Magnetism and the Chemical Bond, Interscience, New York, 1963.
- 11However, note that nonporous densely packed structures were obtained when 1–3 were crystallized in other solvents such as 1,2-dichloroethane/diethyl ether,[7] acetone/diethyl ether, and acetone/toluene. Tetraethylammonium salts of 1–3 or TBA salt of other dimeric polyoxometalates also gave nonporous structures, which showed that the presence of the M2Zn2-substituted POMs (M=Co, Ni, Zn), TBA cations, and EtOAc is essential for the construction of the large hollow structures.
- 12A. L. Spek, PLATON: A Multipurpose Crystallographic Tool, Utrecht University, Utrecht, The Netherlands, 2001.
- 13
- 13aW. Kolodziejski, J. Klinowski, Chem. Rev. 2002, 102, 613–628;
- 13bS. H. Liang, I. D. Gay, Langmuir 1985, 1, 593–599.
- 14L.-Q. Wang, J. Liu, G. J. Exarhos, B. C. Bunker, Langmuir 1996, 12, 2663–2669.
- 15The 1H NMR spectra of the collected sample in [D6]DMSO showed the DMC signal and no EtOAc signals were observed, indicating the exchange of EtOAc with DMC. The amount of DMC per void was estimated by comparison of the integrated signal intensity with that of TBA. It was confirmed by the elemental analyses that the amount of TBA cations was unchanged by the guest-exchange experiments (N contents: before exchange 1.58 wt %, after exchange 1.60 wt %).