Induced-Fit Binding of a Polyproline Helix by a β-Hairpin Peptide†
Dr. Dale J. Wilger
Department of Chemistry, University of North Carolina at Chapel Hill, CB 3290, Chapel Hill, NC 27599 (USA)
Search for more papers by this authorJessica H. Park
Department of Chemistry, University of North Carolina at Chapel Hill, CB 3290, Chapel Hill, NC 27599 (USA)
Search for more papers by this authorDr. Robert M. Hughes
Department of Chemistry, University of North Carolina at Chapel Hill, CB 3290, Chapel Hill, NC 27599 (USA)
Search for more papers by this authorDr. Matthew E. Cuellar
College of Pharmacy, University of Minnesota, 308 Harvard Street SE, Minneapolis, MN 55455 (USA)
Search for more papers by this authorCorresponding Author
Prof. Marcey L. Waters
Department of Chemistry, University of North Carolina at Chapel Hill, CB 3290, Chapel Hill, NC 27599 (USA)
Department of Chemistry, University of North Carolina at Chapel Hill, CB 3290, Chapel Hill, NC 27599 (USA)Search for more papers by this authorDr. Dale J. Wilger
Department of Chemistry, University of North Carolina at Chapel Hill, CB 3290, Chapel Hill, NC 27599 (USA)
Search for more papers by this authorJessica H. Park
Department of Chemistry, University of North Carolina at Chapel Hill, CB 3290, Chapel Hill, NC 27599 (USA)
Search for more papers by this authorDr. Robert M. Hughes
Department of Chemistry, University of North Carolina at Chapel Hill, CB 3290, Chapel Hill, NC 27599 (USA)
Search for more papers by this authorDr. Matthew E. Cuellar
College of Pharmacy, University of Minnesota, 308 Harvard Street SE, Minneapolis, MN 55455 (USA)
Search for more papers by this authorCorresponding Author
Prof. Marcey L. Waters
Department of Chemistry, University of North Carolina at Chapel Hill, CB 3290, Chapel Hill, NC 27599 (USA)
Department of Chemistry, University of North Carolina at Chapel Hill, CB 3290, Chapel Hill, NC 27599 (USA)Search for more papers by this authorThe National Science Foundation is gratefully acknowledged for funding of this research (Grant No. CHE 0716126).
Graphical Abstract
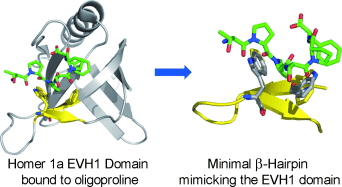
Form-fitting: The study of a minimal mimic of a protein domain that binds to type II polyproline helices through an aromatic cleft is reported. This binding motif mimics that of protein domains, including those important in disease states such as HIV infection and cancer. This study provides insight into the structure–function relationship in binding as well as quantitative data on the magnitude of prolyl–π interactions relevant to inhibitor design.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201106177_sm_miscellaneous_information.pdf1.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1D. Pal, P. Chakrabarti, J. Mol. Biol. 1999, 294, 271–288.
- 2G. Fischer, Chem. Soc. Rev. 2000, 29, 119–127.
- 3W. J. Wedemeyer, E. Welker, H. A. Scheraga, Biochemistry 2002, 41, 14637–14644.
- 4H. Y. Meng, K. M. Thomas, A. E. Lee, N. J. Zondlo, Biopolymers 2006, 84, 192–204.
- 5L. J. Ball, R. Kühne, J. Schneider-Mergener, H. Oschkinat, Angew. Chem. 2005, 117, 2912–2930; Angew. Chem. Int. Ed. 2005, 44, 2852–2869.
- 6A. Zarrinpar, R. P. Bhattacharyya, W. A. Lim, Science’s STKE 2003, 2003, RE 8.
10.1126/stke.2003.179.re8 Google Scholar
- 7C. Freund, H.-G. Schmalz, J. Sticht, R. Kühne, Handb. Exp. Pharmacol. 2008, 186, 407–429.
- 8B. K. Kay, M. P. Williamson, M. Sudol, FASEB J. 2000, 14, 231–241.
- 9J. R. Engen, T. E. Wales, J. M. Hochren, M. A. Meyn III, S. Banu Ozkan, I. Bahar, T. E. Smithgall, Cell. Mol. Life Sci. 2008, 65, 3058–3073.
- 10G. Trigiante, X. Lu, Nat. Rev. Cancer 2006, 6, 217–226.
- 11M. Hiipakka, P. Huotari, A. Manninen, G. H. Renkema, K. Saksela, Virology 2001, 286, 152–159.
- 12J. Zaminer, C. Brockmann, P. Huy, R. Opitz, C. Reuter, M. Beyermann, C. Freund, M. Müller, H. Oschkinat, R. Kühne, H.-G. Schmalz, Angew. Chem. 2010, 122, 7265–7269;
10.1002/ange.201001739 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7111–7115.
- 13A. Goldstrohm, T. Albrecht, C. Suñé, M. Beford, M. Garcia-Blanco, Mol. Cell. Biol. 2001, 21, 7617–7628.
- 14X.-L. Lu, K. Cao, X.-Y. Liu, B.-H. Jiao, Curr. Med. Chem. 2010, 17, 1117–1124.
- 15S. Breuer, S. I. Schievink, A. Schulte, W. Blankenfeldt, O. T. Fackler, M. Geyer, PLoS ONE 2011, 6, e 20033.
- 16A. G. Cochran, N. J. Skelton, M. A. Starovasnik, Proc. Natl. Acad. Sci. USA 2001, 98, 5578–5583.
- 17S. M. Butterfield, M. L. Waters, J. Am. Chem. Soc. 2003, 125, 9580–9581.
- 18S. M. Butterfield, M. M. Sweeny, M. L. Waters, J. Org. Chem. 2005, 70, 1105–1114.
- 19S. M. Butterfield, W. J. Cooper, M. L. Waters, J. Am. Chem. Soc. 2005, 127, 24–25.
- 20P. B. Harbury, T. Zhang, P. S. Kim, T. Alber, Science 1993, 262, 1401–1407.
- 21K. J. Lumb, P. S. Kim, Biochemistry 1995, 34, 8642–8642.
- 22M. G. Oakley, P. S. Kim, Biochemistry 1998, 37, 12603–12610.
- 23J. R. Lai, J. D. Fisk, B. Weisblum, S. H. Gellman, J. Am. Chem. Soc. 2004, 126, 10514–10515.
- 24F. A. Syud, J. F. Espinosa, S. H. Gellman, J. Am. Chem. Soc. 1999, 121, 11577–11578.
- 25F. A. Bovey, F. P. Hood, Biopolymers 1967, 5, 325–326.
- 26S. Brahms, J. Brahms, J. Mol. Biol. 1980, 138, 149–178.
- 27B. W. Chellgren, T. P. Creamer, Biochemistry 2004, 43, 5864–5869.
- 28M. Kuemin, S. Schweizer, C. Ochsenfeld, H. Wennemers, J. Am. Chem. Soc. 2009, 131, 15474–15482.
- 29D. S. Wishart, B. D. Sykes, F. M. Richards, J. Mol. Biol. 1991, 222, 311–333.
- 30K. M. Thomas, D. Naduthambi, N. J. Zondlo, J. Am. Chem. Soc. 2006, 128, 2216–2217.
- 31J. D. Steinkruger, D. N. Woolfson, S. H. Gellman, J. Am. Chem. Soc. 2010, 132, 7586–7588.
- 32L. Roy, M. A. Case, J. Am. Chem. Soc. 2010, 132, 8894–8896.
- 33R. M. Hughes, PhD dissertation, University of North Carolina at Chapel Hill, 2007.