Aerobic Alcohol Oxidations Mediated by Nitric Acid†
Christof Aellig
ETH Zurich, Institute for Chemical and Bioengineering, Wolfgang-Pauli-Strasse 10, CH-8093 Zurich (Switzerland) http://www.hermans.ethz.ch
Search for more papers by this authorChristophe Girard
ETH Zurich, Institute for Chemical and Bioengineering, Wolfgang-Pauli-Strasse 10, CH-8093 Zurich (Switzerland) http://www.hermans.ethz.ch
Search for more papers by this authorCorresponding Author
Prof. Dr. Ive Hermans
ETH Zurich, Institute for Chemical and Bioengineering, Wolfgang-Pauli-Strasse 10, CH-8093 Zurich (Switzerland) http://www.hermans.ethz.ch
ETH Zurich, Institute for Chemical and Bioengineering, Wolfgang-Pauli-Strasse 10, CH-8093 Zurich (Switzerland) http://www.hermans.ethz.chSearch for more papers by this authorChristof Aellig
ETH Zurich, Institute for Chemical and Bioengineering, Wolfgang-Pauli-Strasse 10, CH-8093 Zurich (Switzerland) http://www.hermans.ethz.ch
Search for more papers by this authorChristophe Girard
ETH Zurich, Institute for Chemical and Bioengineering, Wolfgang-Pauli-Strasse 10, CH-8093 Zurich (Switzerland) http://www.hermans.ethz.ch
Search for more papers by this authorCorresponding Author
Prof. Dr. Ive Hermans
ETH Zurich, Institute for Chemical and Bioengineering, Wolfgang-Pauli-Strasse 10, CH-8093 Zurich (Switzerland) http://www.hermans.ethz.ch
ETH Zurich, Institute for Chemical and Bioengineering, Wolfgang-Pauli-Strasse 10, CH-8093 Zurich (Switzerland) http://www.hermans.ethz.chSearch for more papers by this authorWe acknowledge the financial support from ETH Zurich (grant ETH-18 09-2).
Graphical Abstract
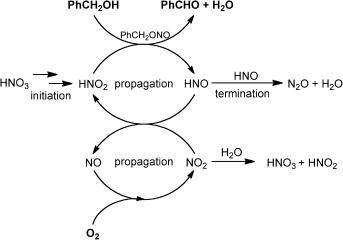
A touch of acid: Catalytic amounts of HNO3 can trigger the aerobic oxidation of alcohols in the presence of the solid acid amberlyst-15. The desired oxidation cycle, mediated by (H)NOx species, is in kinetic competition with the detrimental formation of N2O by HNO dimerization (see scheme). In situ water removal in a gas recirculation reactor increases the reaction rate and the end-conversion by minimizing N2O formation and increasing the (H)NOx turnover.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201105620_sm_miscellaneous_information.pdf105.2 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1F. Cavani, J. H. Teles, ChemSusChem 2009, 2, 508–534.
- 2G. Franz, R. Sheldon, Oxidation, Ullmann’s Encycl. Ind. Chem., Wiley-VCH, Weinheim, 2000.
- 3See, for example,
- 3aI. Hermans, T. L. Nguyen, P. A. Jacobs, J. Peeters, ChemPhysChem 2005, 6, 637–645;
- 3bI. Hermans, P. A. Jacobs, J. Peeters, Chem. Eur. J. 2006, 12, 4229–4240;
- 3cU. Neuenschwander, F. Guignard, I. Hermans, ChemSusChem 2010, 3, 75–84.
- 4M. Thiemann, E. Scheibler, K. W. Wiegand, Nitric Acid, Nitrous Acid, and Nitrogen Oxides, Ullmann’s Encycl. Ind. Chem., Wiley-VCH, Weinheim, 2000.
- 5C. Seidel, BASF expands methanesulfonic acid plant in Ludwigshafen, Press Information, May 11th 2010.
- 6See, for example, H. Osato, M. Kabaki, S. Shimizu, Org. Process Res. Dev. 2011, 15, 581–584, and references therein.
- 7M. T. Musser, Adipic Acid, Ullmann’s Encycl. Ind. Chem., Wiley-VCH, Weinheim, 2000.
- 8
- 8aI. Hermans, K. Janssen, B. Moens, A. Philippaerts, B. Van Berlo, J. Peeters, P. A. Jacobs, B. F. Sels, Adv. Syn. Catal. 2007, 349, 1604–1608, and references therein;
- 8bK. Banert, O. Plefka, Angew. Chem. 2011, 123, 6295–6298;
10.1002/ange.201101326 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 6171–6174.
- 9A. Levina, S. Trusov, J. Mol. Catal. 1994, 88, L121–L123.
- 10aY. Kuang, N. M. Islam, Y. Nabae, T. Hayakawa, M. Kakimoto, Angew. Chem. 2010, 122, 446–450; Angew. Chem. Int. Ed. 2010, 49, 436–440;
- 10bY. Kuang, H. Rokubuichi, Y. Nabae, T. Hayakawa, M. Kakimoto, Adv. Synth. Catal. 2010, 352, 2635–2642.
- 11
- 11aF. Bleger, O. Simon, A. Schouteeten, WO 2009/092734, 2009;
- 11bF. Bleger, O. Simon, A. Schouteeten, US 2011/0012056A1, 2011.
- 12
- 12aY. Ogata, Y. Sawaki, F. Matsunaga, H. Tezuka, Tetrahedron 1966, 22, 2655–2664;
- 12bD. S. Ross, C.-L. Gu, G. P. Hum, R. Malhotra, Int. J. Chem. Kinet. 1986, 18, 1277–1288;
- 12cS. R. Joshi, K. L. Kataria, S. B. Sawant, J. B. Joshi, Ind. Eng. Chem. Res. 2005, 44, 325–333.
- 13I. Hermans, J. Peeters, L. Vereecken, P. Jacobs, ChemPhysChem 2007, 8, 2678–2688.
- 14Y. Wada, K. Morimoto, T. Goibuchi, H. Tomiyasu, Radiochim. Acta 1995, 68, 233–243.
- 15A. I. Kazakov, Yu. I. Rubtsov, L. P. Andrienko, G. B. Manelis, Russ. Chem. Bull. 1987, 36, 1999–2002.
- 16Y. Kameoka, R. L. Pigford, Ind. Eng. Chem. Fundam. 1977, 16, 163–169.
- 17K. P. C. Vollhardt, N. E. Shore, Organic Chemistry Structure and Function, 4th ed., Freeman, New York, 2002.
- 18M. A. Beckett, I. Hua, Environ. Sci. Technol. Environ. Sci. Technol. 2000, 34, 3944–3953.
- 19M. P. Doyle, J. W. Terpstra, R. A. Pickering, D. M. LePoire, J. Org. Chem. 1983, 48, 3379–3382.
- 20H. F. Cordes, N. R. Fetter, J. A. Happe, J. Am. Chem. Soc. 1958, 80, 4802–4808.
- 21V. Shafirovich, V. S. Lymar, Proc. Natl. Acad. Sci. USA 2002, 99, 7340–7345.
- 22NIST Chemical Kinetics Database, http://kinetics.nist.gov/kinetics/.
- 23W. Tsang, J. T. Herron, J. Phys. Chem. Ref. Data 1991, 20, 609–663.
- 24R. Svensson, E. Ljungström, Int. J. Chem. Kinet. 1988, 20, 857–866.