Total Synthesis of Spirastrellolide F Methyl Ester—Part 2: Macrocyclization and Completion of the Synthesis†
Stefan Benson Dipl.-Chem.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorMarie-Pierre Collin Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorGregory W. O'Neil Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorJulien Ceccon Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorBernhard Fasching Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorMichaël D. B. Fenster Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorCédrickx Godbout Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorKarin Radkowski
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorRichard Goddard Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorAlois Fürstner Prof.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorStefan Benson Dipl.-Chem.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorMarie-Pierre Collin Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorGregory W. O'Neil Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorJulien Ceccon Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorBernhard Fasching Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorMichaël D. B. Fenster Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorCédrickx Godbout Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorKarin Radkowski
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorRichard Goddard Dr.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorAlois Fürstner Prof.
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim/Ruhr (Germany), Fax: (+49) 208-306-2994
Search for more papers by this authorGenerous financial support by the MPG, the Chemical Genomics Center (CGC) of the MPG, the Fonds der Chemischen Industrie (Kekulé-stipend to S.B.), the Alexander-von-Humboldt Foundation (fellowships to G.O’N. and M.D.B.F.), the Fonds de Recherche sur la Nature et les Technologies Québec (fellowship to C.G.), and F. Hoffmann–La Roche, Basel, is gratefully acknowledged. We sincerely thank the NMR, X-ray, and chromatography departments of our Institute for excellent support over the entire duration of this project, the ANKA Angstroemquelle, Karlsruhe, for the provision of beamtime, and Prof. R. J. Andersen, University of British Columbia, Vancouver, for an exchange of information.
Graphical Abstract
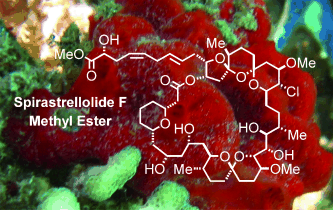
Marvel of the sea: A concise and highly convergent total synthesis of the methyl ester of the marine macrolide spirastrellolide F (see picture), which has exquisite antimitotic properties, is reported. In this approach, the northern and the southern hemispheres of this intricate target are stitched together in only two consecutive steps (Suzuki coupling, Yamaguchi lactonization) without any interim protecting-group manipulations.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200906122_sm_miscellaneous_information.pdf595.1 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. E. Williams, M. Roberge, R. Van Soest, R. J. Andersen, J. Am. Chem. Soc. 2003, 125, 5296–5297;
- 1bD. E. Williams, M. Lapawa, X. Feng, T. Tarling, M. Roberge, R. J. Andersen, Org. Lett. 2004, 6, 2607–2610;
- 1cD. E. Williams, R. A. Keyzers, K. Warabi, K. Desjardine, J. L. Riffell, M. Roberge, R. J. Andersen, J. Org. Chem. 2007, 72, 9842–9845;
- 1dK. Warabi, D. E. Williams, B. O. Patrick, M. Roberge, R. J. Andersen, J. Am. Chem. Soc. 2007, 129, 508–509.
- 2Review: I. Paterson, S. M. Dalby, Nat. Prod. Rep. 2009, 26, 865–873.
- 3aI. Paterson, E. A. Anderson, S. M. Dalby, O. Loiseleur, Org. Lett. 2005, 7, 4121–4224;
- 3bI. Paterson, E. A. Anderson, S. M. Dalby, O. Loiseleur, Org. Lett. 2005, 7, 4125–4128;
- 3cJ. Liu, R. P. Hsung, Org. Lett. 2005, 7, 2273–2276;
- 3dY. Pan, J. K. De Brabander, Synlett 2006, 853–856;
- 3eI. Paterson, E. A. Anderson, S. M. Dalby, J. H. Lim, P. Maltas, C. Moessner, Chem. Commun. 2006, 4186–4188;
- 3fC. Wang, C. J. Forsyth, Org. Lett. 2006, 8, 2997–3000;
- 3gJ. Liu, J. H. Yang, C. Ko, R. P. Hsung, Tetrahedron Lett. 2006, 47, 6121–6123;
- 3hI. Paterson, E. A. Anderson, S. M. Dalby, J. Genovino, J. H. Lim, C. Moessner, Chem. Commun. 2007, 1852–1854;
- 3iA. B. Smith, D.-S. Kim, Org. Lett. 2007, 9, 3311–3314;
- 3jC. Wang, C. J. Forsyth, Heterocycles 2007, 72, 621–632;
- 3kK. A. Keaton, A. J. Phillips, Org. Lett. 2008, 10, 1083–1086;
- 3lS. Chandrasekhar, C. Rambabu, A. S. Reddy, Org. Lett. 2008, 10, 4355–4357;
- 3mJ.-H. Yang, J. Liu, R. P. Hsung, Org. Lett. 2008, 10, 2525–2528.
- 4aA. Fürstner, M. D. B. Fenster, B. Fasching, C. Godbout, K. Radkowski, Angew. Chem. 2006, 118, 5632–5636;
10.1002/ange.200601654 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5506–5510;
- 4bA. Fürstner, M. D. B. Fenster, B. Fasching, C. Godbout, K. Radkowski, Angew. Chem. 2006, 118, 5636–5641;
10.1002/ange.200601655 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5510–5515.
- 5A. Fürstner, B. Fasching, G. W. O’Neil, M. D. B. Fenster, C. Godbout, J. Ceccon, Chem. Commun. 2007, 3045–3047.
- 6
- 6aI. Paterson, E. A. Anderson, S. M. Dalby, J. H. Lim, J. Genovino, P. Maltas, C. Moessner, Angew. Chem. 2008, 120, 3058–3062; Angew. Chem. Int. Ed. 2008, 47, 3016–3020;
- 6bI. Paterson, E. A. Anderson, S. M. Dalby, J. H. Lim, J. Genovino, P. Maltas, C. Moessner, Angew. Chem. 2008, 120, 3063–3067; Angew. Chem. Int. Ed. 2008, 47, 3021–3025.
- 7G. W. O’Neil, J. Ceccon, S. Benson, M.-P. Collin, B. Fasching, A. Fürstner, Angew. Chem. 2009, 121, 10124–10129; Angew. Chem. Int. Ed. 2009, 48, 9940–9945.
- 8
- 8aR. W. Hoffmann, Synthesis 2006, 3531–3541;
- 8bI. S. Young, P. S. Baran, Nat. Chem. 2009, 1, 193–205;
- 8cA. Fürstner, Synlett 1999, 1523–1533.
- 9
- 9aN. Miyaura, T. Ishiyama, H. Sasaki, M. Ishikawa, M. Satoh, A. Suzuki, J. Am. Chem. Soc. 1989, 111, 314–321;
- 9breview: S. R. Chemler, D. Trauner, S. J. Danishefsky, Angew. Chem. 2001, 113, 4676–4701;
10.1002/1521-3757(20011217)113:24<4676::AID-ANGE4676>3.0.CO;2-B Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4544–4568.10.1002/1521-3773(20011217)40:24<4544::AID-ANIE4544>3.0.CO;2-N CAS PubMed Web of Science® Google Scholar
- 10The antiperiplanar conformation of the unprotected diol is evident from the 3JH22,H23 value of the natural product and a crystal structure reported by Paterson et al. (Ref. [6b]). It was assumed that silylation of O22 and O23 does not change this arrangement.
- 11J. Inanaga, K. Hirata, H. Saeki, T. Katsuki, M. Yamaguchi, Bull. Chem. Soc. Jpn. 1979, 52, 1989–1993.
- 12
- 12aReview: A. Parenty, X. Moreau, J.-M. Campagne, Chem. Rev. 2006, 106, 911–939;
- 12bfor applications in natural product synthesis from this laboratory, see J. Mlynarski, J. Ruiz-Caro, A. Fürstner, Chem. Eur. J. 2004, 10, 2214–2222;
- 12cA. Fürstner, E. Kattnig, O. Lepage, J. Am. Chem. Soc. 2006, 128, 9194–9204;
- 12dA. Fürstner, C. Aïssa, C. Chevrier, F. Teplý, C. Nevado, M. Tremblay, Angew. Chem. 2006, 118, 5964–5969;
10.1002/ange.200601859 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5832–5837;
- 12eA. Fürstner, E. Kattnig, G. Kelter, H.-H. Fiebig, Chem. Eur. J. 2009, 15, 4030–4043.
- 13In addition to Pd(OH)2, the following (pre)catalysts were screened for the reduction of compounds 9 and/or 10 in different solvents using hydrogen pressures ranging from 1–200 atm at different temperatures (RT→100 °C), with or without ultrasonication to enhance reactivity: Pd/C, Pd black, Rh/C, Rh/Al2O3, Ir black, PtO2, [RhCl(Ph3P)3]; moreover, reductions were attempted with HOOC-NN-COOK/HOAc, o-NO2-C6H4-SO2NH-NH2/Et3N, Cy2BH/HOAc (Cy=cyclohexyl).
- 14The distance from H13 to the centroid of the CC bond is only 2.794 Å and the C13⋅⋅⋅C58 distance is remarkably short (3.61 Å) in spite of a C13-H13-C.58 angle of 158°. There are no crystal structures in the Cambridge Database (September 2009) containing similar structural elements that have shorter intermolecular C⋅⋅⋅C distances with such a large C-H angle. Furthermore, the C13H13 bond is calculated to be only 1.001 Å.
- 15For the sake of clarity, anisotropic displacement parameters are drawn only at the 5 % (10) and 20 % (12) probability level. CCDC 749791 (10) and 752194 (12) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Additional views of the structures and short crystallographic summaries are also found in the Supporting Information.
- 16R. Crabtree, Acc. Chem. Res. 1979, 12, 331–337.
- 17The use of a weakly coordinating BARF counterion is thought to foster substrate binding, increase the catalyst lifetime, and reduce formation of the iridium hydride trimer, which is one of the prominent decompositions pathways of such catalysts, see
- 17aS. P. Smidt, A. Pfaltz, E. Martinez-Viviente, P. S. Pregosin, A. Albinati, Organometallics 2003, 22, 1000–1009;
- 17bY. Xu, D. M. P. Mingos, J. M. Brown, Chem. Commun. 2008, 199–201.
- 18This propensity enables directed hydrogenations, see
- 18aJ. M. Brown, Angew. Chem. 1987, 99, 169–182; Angew. Chem. Int. Ed. Engl. 1987, 26, 190–203;
- 18bA. H. Hoveyda, D. A. Evans, G. C. Fu, Chem. Rev. 1993, 93, 1307–1370; moreover, it is known that THF and other ethereal solvents strongly alter the reactivity of cationic iridium hydrogenation catalysts, see
- 18cM. Inoue, M. Nakada, J. Am. Chem. Soc. 2007, 129, 4164–4165;
- 18dJ. Cipot, R. McDonald, M. J. Ferguson, G. Schatte, M. Stradiotto, Organometallics 2007, 26, 594–608.
- 19B. Fasching, Dissertation, Technische Universität Dortmund, 2007.
- 20A. K. Chatterjee, T.-L. Choi, D. P. Sanders, R. H. Grubbs, J. Am. Chem. Soc. 2003, 125, 11360–11370.
- 21The following example is representative (Ref. [19]):
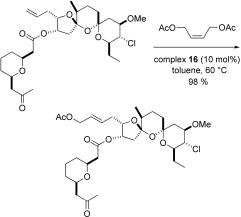 The reasons for the strikingly different ease, with which this model and compound 14 undergo CM, remain unclear. Preliminary data, however, suggest that the oxygen atom of the tetrahydrofuran ring in 14 coordinates with the Ru center and hence likely interferes with the performance of the metathesis catalyst, see A. Fürstner, K. Langemann, J. Am. Chem. Soc. 1997, 119, 9130–9136. Details will be reported in a future full paper.
The reasons for the strikingly different ease, with which this model and compound 14 undergo CM, remain unclear. Preliminary data, however, suggest that the oxygen atom of the tetrahydrofuran ring in 14 coordinates with the Ru center and hence likely interferes with the performance of the metathesis catalyst, see A. Fürstner, K. Langemann, J. Am. Chem. Soc. 1997, 119, 9130–9136. Details will be reported in a future full paper.
- 22The optimum conditions were those described in the following paper, see C. D. Vanderwal, D. A. Vosburg, S. Weiler, E. J. Sorensen, J. Am. Chem. Soc. 2003, 125, 5393–5407.
- 23In our previous study, we have described a donor analogous to 18 protected by a TBS group. It was found, however, that this particular silyl group cleaves much slower than the secondary TBS groups at O9 and O1 as well as the isopropylidene acetal. Prolonged stirring of the material with PPTS, however, leads to partial decomposition of the final product. Therefore, the donor fragment 18 was prepared, which carries a more labile TES group as shown in Scheme 3. Furthermore, attempted coupling with the analogous donor featuring a free hydroxy group, as used by Paterson et al.,[6] did not work well in our hands.
- 24For details and a comprehensive data set, see the Supporting Information.
- 25It is of note that the NMR spectrum of synthetic spirastrellolide A methyl ester reported by Paterson and coworkers is identical to that of an authentic sample recorded under the same conditions on the same spectrometer,[6] but is distinctly different from the spectrum depicted in the Supporting Information of the isolation paper.[1]