Non-Metal-Mediated Homogeneous Hydrogenation of CO2 to CH3OH†
Andrew E. Ashley Dr.
Chemistry Research Laboratory, University of Oxford, Mansfield Road, Oxford OX1 3TA (UK), Fax: (+44) 1865-272-690
Search for more papers by this authorAmber L. Thompson Dr.
Chemical Crystallography, Inorganic Chemistry Laboratory, South Parks Road, Oxford OX1 3QR (UK)
Search for more papers by this authorDermot O'Hare Prof.
Chemistry Research Laboratory, University of Oxford, Mansfield Road, Oxford OX1 3TA (UK), Fax: (+44) 1865-272-690
Search for more papers by this authorAndrew E. Ashley Dr.
Chemistry Research Laboratory, University of Oxford, Mansfield Road, Oxford OX1 3TA (UK), Fax: (+44) 1865-272-690
Search for more papers by this authorAmber L. Thompson Dr.
Chemical Crystallography, Inorganic Chemistry Laboratory, South Parks Road, Oxford OX1 3QR (UK)
Search for more papers by this authorDermot O'Hare Prof.
Chemistry Research Laboratory, University of Oxford, Mansfield Road, Oxford OX1 3TA (UK), Fax: (+44) 1865-272-690
Search for more papers by this authorWe thank the EPSRC for support, Balliol College, Oxford for a Junior Research Fellowship (A.E.A.), and Dr. Nick Rees (CRL, Oxford) for NMR support.
Graphical Abstract
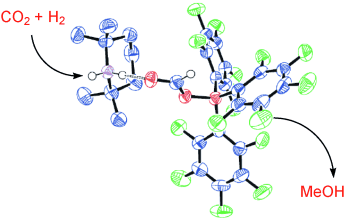
Turning a problem to advantage: CO2, a contributor to global warming, was converted into the valuable resource CH3OH by adding it to 2,2,6,6-tetramethylpiperidine and B(C6F5)3 in toluene under H2 (1–2 atm), heating the mixture at 160 °C, and vacuum distillation. CH3OH was formed via the complex shown (C blue, N purple, O red, B orange, F green) as the sole C1 product.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200905466_sm_miscellaneous_information.pdf43.4 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1ahttp://www.co2science.org/;
- 1bJ. Hansen, L. Nazarenko, R. Ruedy, M. Sato, J. Willis, A. Del Genio, D. Koch, A. Lacis, K. Lo, S. Menon, T. Novakov, J. Perlwitz, G. Russell, G. A. Schmidt, N. Tausnev, Science 2005, 308, 1431–1451.
- 2
- 2aK. B. Lee, M. G. Beaver, H. S. Caram, S. Sircar, Ind. Eng. Chem. Res. 2008, 47, 8048–8062;
- 2bA. Demessence, D. M. D’Alessandro, M. L. Foo, J. R. Long, J. Am. Chem. Soc. 2008, 131, 8048–8062;
- 2cA. J. Fletcher, E. J. Cussen, T. J. Prior, M. J. Rosseinsky, C. J. Kepert, K. M. Thomas, J. Am. Chem. Soc. 2001, 123, 10001–10011;
- 2dS. Surblé, F. Millange, C. Serre, T. Düren, M. Latroche, S. Bourrelly, P. L. Llewellyn, G. Férey, J. Am. Chem. Soc. 2006, 128, 14889–14896;
- 2eR. Vaidhyanathan, S. S. Iremonger, K. W. Dawson, G. K. H. Shimizu, Chem. Commun. 2009, 5230–5232.
- 3P. G. Jessop in Handbook of Homogeneous Hydrogenation, Vol. 1 (Eds.: ), Wiley-VCH, Weinheim, 2007, pp. 489–511; for reviews, see:
- 3aP. G. Jessop, T. Ikariya, R. Noyori, Chem. Rev. 1995, 95, 259–272;
- 3bP. G. Jessop, F. Joo, C.-C. Tai, Coord. Chem. Rev. 2004, 248, 2425–2442;
- 3cT. Sakakura, J.-C. Choi, H. Yasuda, Chem. Rev. 2007, 107, 2365–2387.
- 4
- 4aK. Tominaga, Y. Sasaki, T. Watanabe, M. Saito, Bull. Chem. Soc. Jpn. 1995, 68, 2837–2842;
- 4bK. Tominaga, Y. Sasaki, M. Kawai, T. Watanabe, M. Saito, J. Chem. Soc. Chem. Commun. 1993, 629–631.
- 5For a recent review, see: D. W. Stephan, Dalton Trans. 2009, 3129–3136.
- 6
- 6aC. Jiang, O. Blacque, H. Berke, Organometallics 2009, 28, 5233–5239;
- 6bD. Holschumacher, C. Taouss, T. Bannenberg, C. G. Hrib, C. G. Daniliuc, P. G. Jones, M. Tamm, Dalton Trans. 2009, 6927–6929;
- 6cA. Ramos, A. J. Lough, D. W. Stephan, Chem. Commun. 2009, 1118–1120;
- 6dV. Sumerin, F. Schulz, M. Atsumi, C. Wang, M. Nieger, M. Leskelä, T. Repo, P. Pyykkö, B. Rieger, J. Am. Chem. Soc. 2008, 130, 14117–14119;
- 6eG. C. Welch, D. W. Stephan, J. Am. Chem. Soc. 2007, 129, 1880–1881;
- 6fP. A. Chase, D. W. Stephan, Angew. Chem. 2008, 120, 7543–7547;
10.1002/ange.200802596 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7433–7437;
- 6gD. Holschumacher, T. Bannenberg, C. G. Hrib, P. G. Jones, M. Tamm, Angew. Chem. 2008, 120, 7538–7542;
10.1002/ange.200802705 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7428–7432;
- 6hD. P. Huber, G. Kehr, K. Bergander, R. Froehlich, G. Erker, S. Tanino, Y. Ohki, K. Tatsumi, Organometallics 2008, 27, 5279–5284;
- 6iG. C. Welch, R. R. S. Juan, J. D. Masuda, D. W. Stephan, Science 2006, 314, 1124–1126; for a review, see:
A. L. Kenward, W. E. Piers, Angew. Chem. 2008, 120, 38–42;
10.1002/ange.200702816 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 38–41; for an example of the ruthenium-mediated heterolytic cleavage of H2, see: M. Ito, T. Ikariya, Chem. Commun. 2007, 5134–5142.
- 7
- 7aK. V. Axenov, G. Kehr, R. Froehlich, G. Erker, Organometallics 2009, 28, 5148–5158;
- 7bC. M. Mömming, S. Froemel, G. Kehr, R. Froehlich, S. Grimme, G. Erker, J. Am. Chem. Soc. 2009, 131, 12280–12289;
- 7cM. Dureen, D. W. Stephan, J. Am. Chem. Soc. 2009, 131, 8396–8397;
- 7dM. Ullrich, K. S. H. Kelvin, A. J. Lough, D. W. Stephan, Chem. Commun. 2009, 2335–2337;
- 7eH. Wang, R. Froehlich, G. Kehr, G. Erker, Chem. Commun. 2008, 5966–5968;
- 7fP. Spies, S. Schwendemann, S. Lange, G. Kehr, R. Froehlich, G. Erker, Angew. Chem. 2008, 120, 7654–7657;
10.1002/ange.200801432 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7543–7546;
- 7gJ. S. J. McCahill, G. C. Welch, D. W. Stephan, Angew. Chem. 2007, 119, 5056–5059;
10.1002/ange.200701215 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 4968–4971;
- 7hP. A. Chase, G. C. Welch, T. Jurca, D. W. Stephan, Angew. Chem. 2007, 119, 8196–8199;
10.1002/ange.200702908 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8050–8053.
- 8C. M. Mömming, E. Otten, G. Kehr, R. Fröhlich, S. Grimme, D. W. Stephan, G. Erker, Angew. Chem. 2009, 121, 6770–6773;
10.1002/ange.200901636 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 6643–6646.
- 9V. Sumerin, F. Schulz, M. Nieger, M. Leskela, T. Repo, B. Rieger, Angew. Chem. 2008, 120, 6090–6092;
10.1002/ange.200800935 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6001–6003.
- 10Even at room temperature (CO2, 1 atm), this reaction proceeded to 18 % conversion in 12 h. No indication of the reaction of 1 with CO was observed at temperatures up to 130 °C. Interestingly, the system [tBu3PH][BH(C6F5)3][6e] does not react with CO2 under the conditions successful for 1.
- 11A similar effect was demonstrated by a 19F/1H NOE experiment: J. M. Blackwell, W. E. Piers, M. Parvez, Org. Lett. 2000, 2, 695–698.
- 12The crystal structure of 2 was solved by direct methods (SIR92) and refined by full-matrix least squares (CRYSTALS). Crystal data for 2: C28H21BF15NO2, Mr=699.26, crystal size: 0.06×0.10×0.10 mm3, triclinic,
 , a=11.6181(1), b=14.9808(2), c=17.5277(2) Å, α=91.3525(5), β=107.8912(6), γ=100.6911(6)°, V=2842.13(6) Å3, Z=4, ρcalcd=1.634 g cm−3, μ=0.169 mm−1, MoKα radiation (λ=0.71073 Å), T=150 K, 2θmax=27.57°, 54 455 measured reflections (12 965 independent, Rint=0.068), absorption correction (semiempirical from equivalents), transmission factors: 0.94/0.99, R=0.0393, wR=0.0904 refined against |F2|, GOF=0.9374, [Δρ]max=0.44, [Δρ]min=−0.41 e Å−3. CCDC 749113 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
, a=11.6181(1), b=14.9808(2), c=17.5277(2) Å, α=91.3525(5), β=107.8912(6), γ=100.6911(6)°, V=2842.13(6) Å3, Z=4, ρcalcd=1.634 g cm−3, μ=0.169 mm−1, MoKα radiation (λ=0.71073 Å), T=150 K, 2θmax=27.57°, 54 455 measured reflections (12 965 independent, Rint=0.068), absorption correction (semiempirical from equivalents), transmission factors: 0.94/0.99, R=0.0393, wR=0.0904 refined against |F2|, GOF=0.9374, [Δρ]max=0.44, [Δρ]min=−0.41 e Å−3. CCDC 749113 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 13S. Mitu, M. C. Baird, Can. J. Chem. 2006, 84, 225.
- 14The heating of a solution of [TMPH][HCO2] in toluene alone resulted in no change; thus, the borane adduct is necessary for further transformations. The Keq value for 2⇌1 was not determined owing to the uncertainty in determining the partitioning of the CO2 between the liquid and gas phases.
- 15
- 15aD. Donghi, D. Maggioni, T. Beringhelli, G. D’Alfonso, P. Mercandelli, A. Sironi, Eur. J. Inorg. Chem. 2008, 1645–1653;
- 15bS. P. Lewis, J. Chai, S. Collins, T. J. J. Sciarone, L. D. Henderson, C. Fan, M. Parvez, W. E. Piers, Organometallics 2009, 28, 249–263.
- 16
- 16aJ. Jones in Core Carbonyl Chemistry, 3rd ed. ), OUP, Oxford, 1997;
- 16bM. B. Smith, J. March in March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Stucture, 5th ed., Wiley-Interscience, New York, 2001, pp. 465–468.
- 17
- 17aC. Bergquist, T. Fillebeen, M. M. Morlok, G. Parkin, J. Am. Chem. Soc. 2003, 125, 6189–6199;
- 17bT. Beringhelli, D. Maggioni, G. D’Alfonso, Organometallics 2001, 20, 4927–4938;
- 17cL. H. Doerrer, M. L. H. Green, J. Chem. Soc. Dalton Trans. 1999, 24, 4325–4329.
- 18H.-J. Frohn, N. Y. Adonin, V. V. Bardin, V. F. Starichenko, Z. Anorg. Allg. Chem. 2002, 628, 2827–2833.
- 19
- 19aA. Di Saverio, F. Focante, I. Camurati, L. Resconi, T. Beringhelli, G. D’Alfonso, D. Donghi, D. Maggioni, P. Mercandelli, A. Sironi, Inorg. Chem. 2005, 44, 5030–5041;
- 19bA. A. Danopoulos, J. R. Galsworthy, M. L. H. Green, L. H. Doerrer, S. Cafferkey, M. B. Hursthouse, Chem. Commun. 1998, 2529–2530.
- 20C. Bergquist, B. M. Bridgewater, C. J. Harlan, J. R. Norton, R. A. Friesner, G. Parkin, J. Am. Chem. Soc. 2000, 122, 10581–10590.
- 21NMR spectroscopy and mass spectrometry (EI) of the residue after distillation to establish the identity of the boron-containing products proved inconclusive; investigations are ongoing.
- 22C. Wang, G. Erker, G. Kehr, K. Wedeking, R. Froehlich, Organometallics 2005, 24, 4760–4773.
- 23L.-L. Huang, M.-H. Xu, G.-Q. Lin, J. Am. Chem. Soc. 2006, 128, 5624–5625.